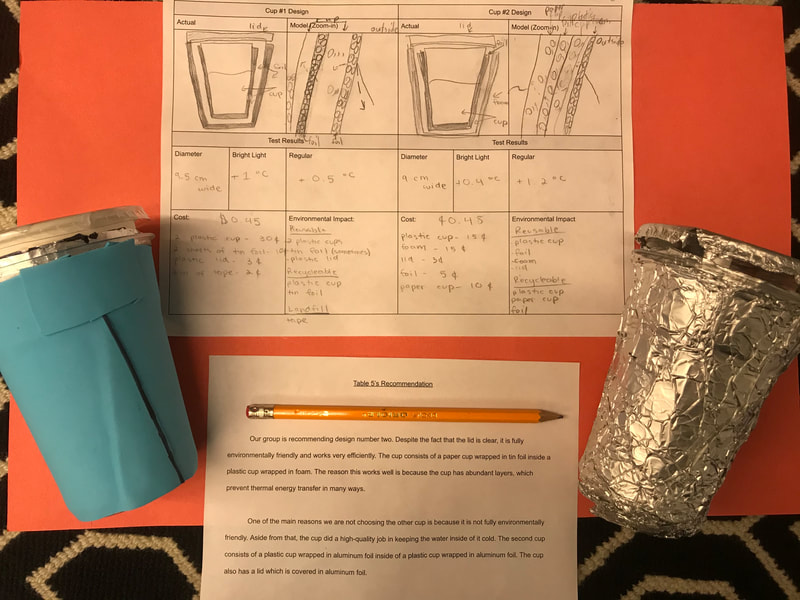
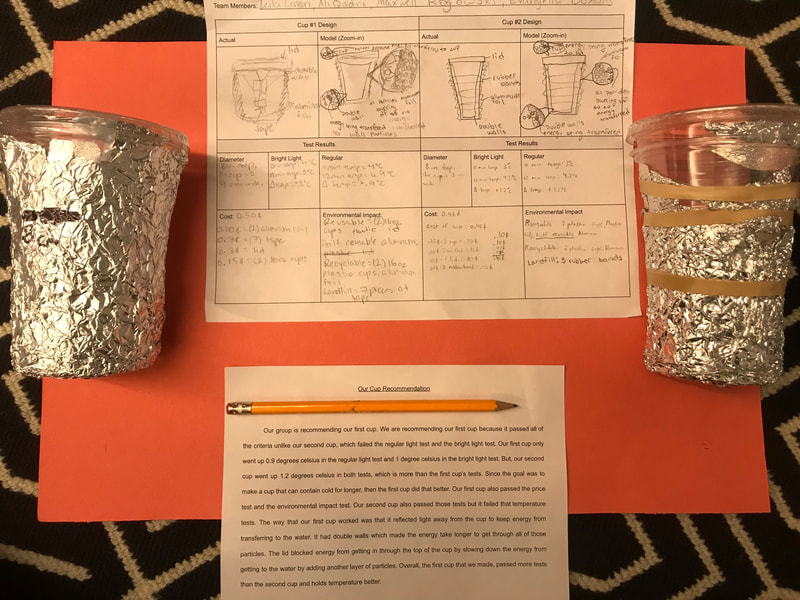
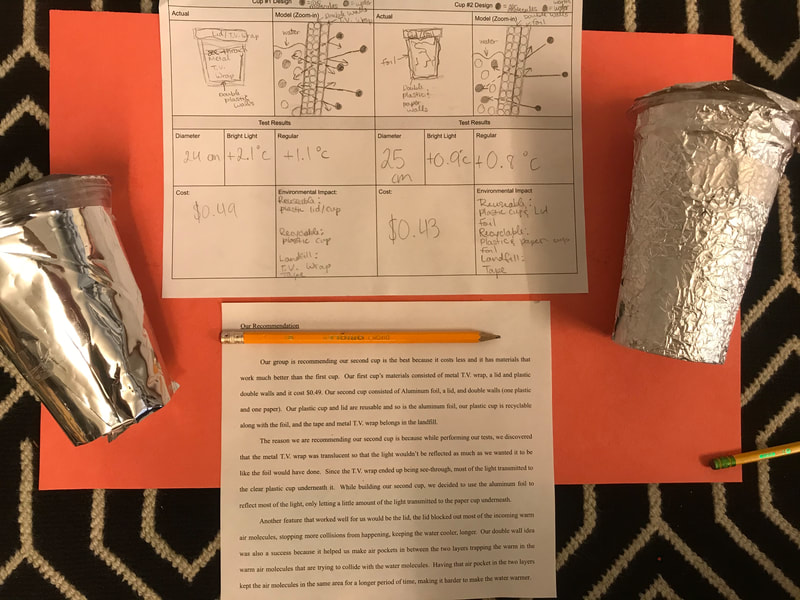
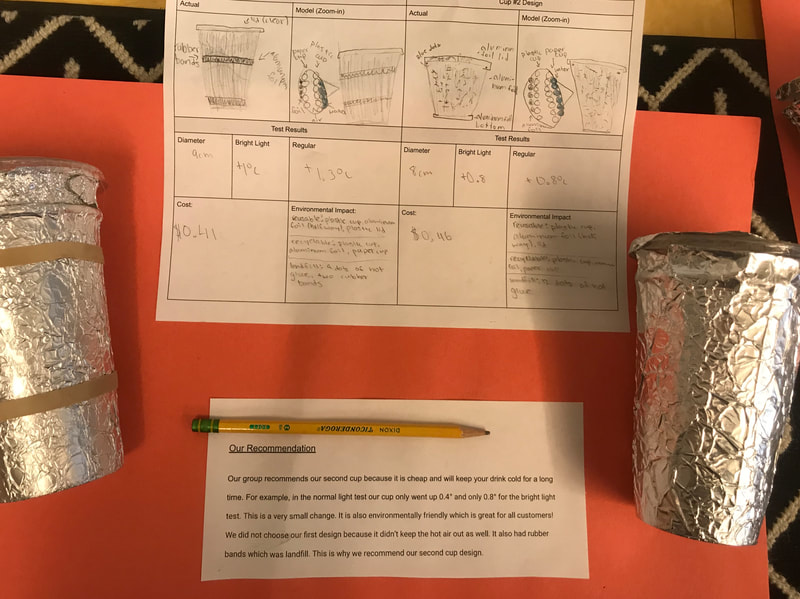
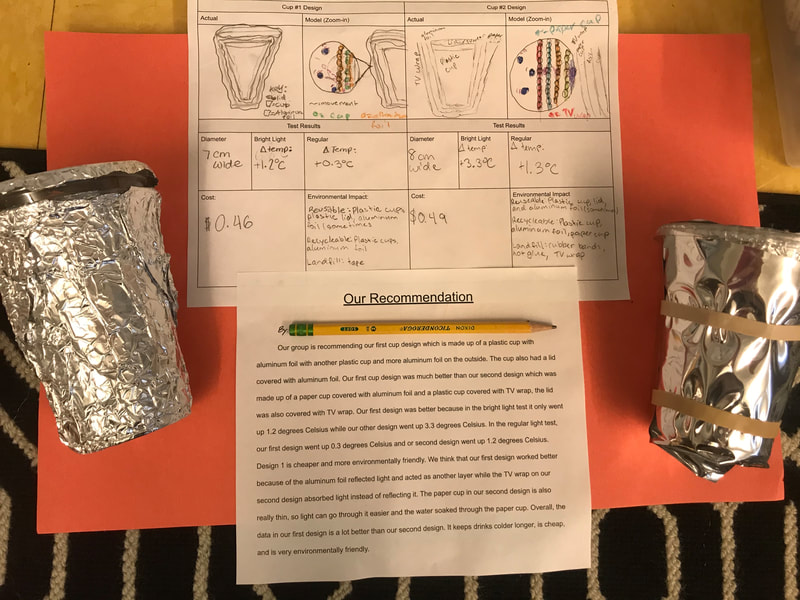
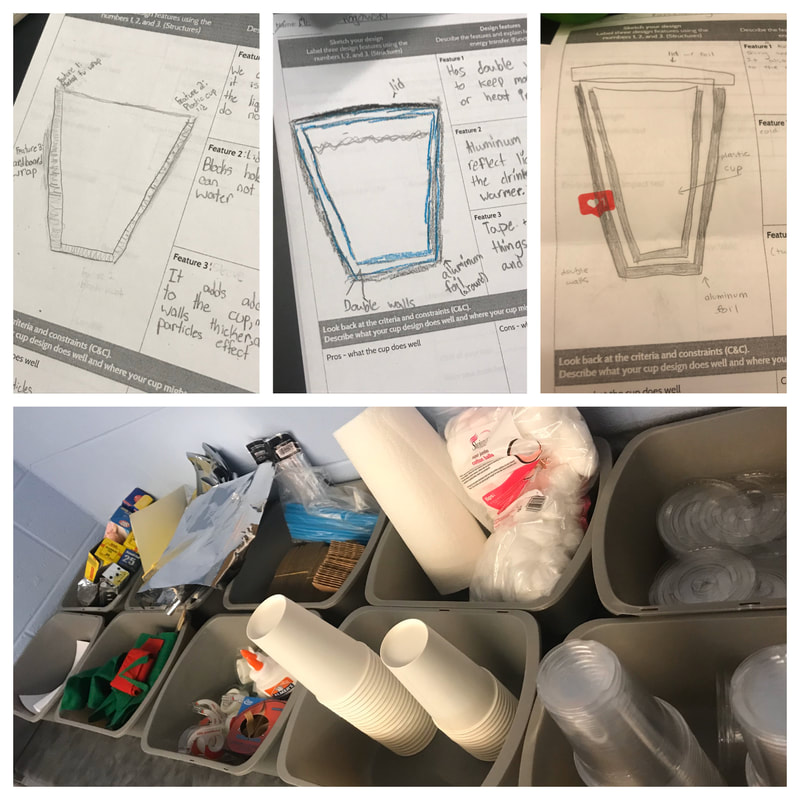
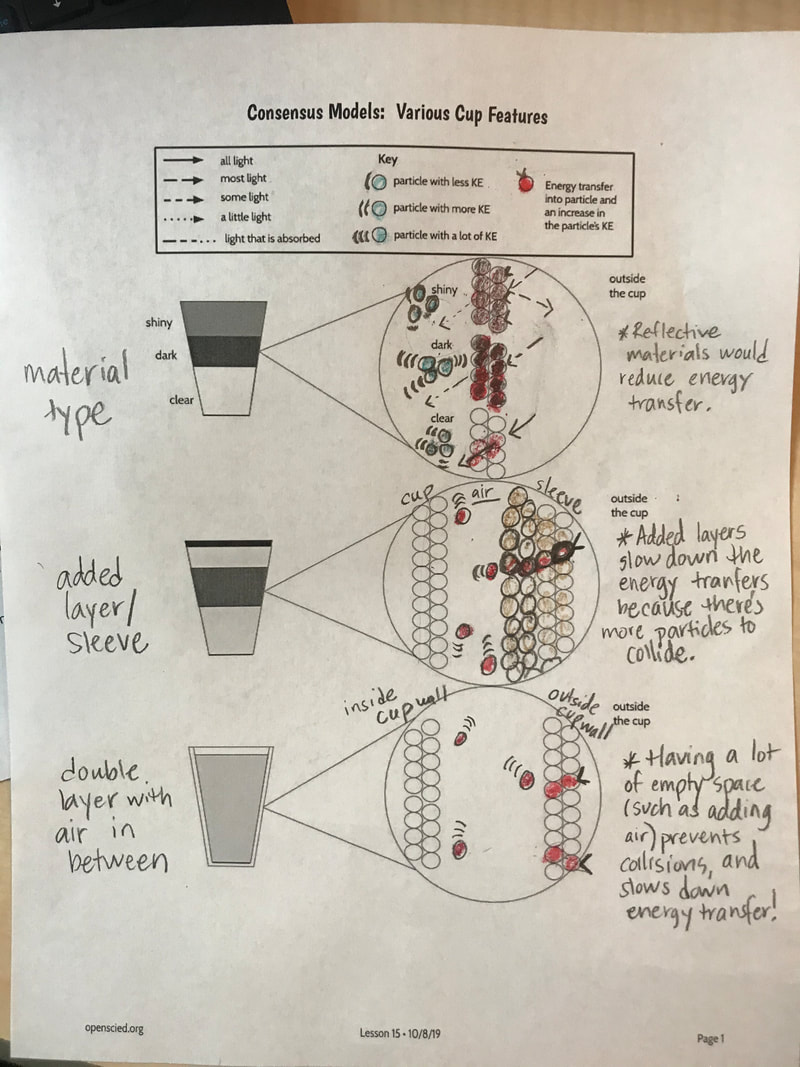
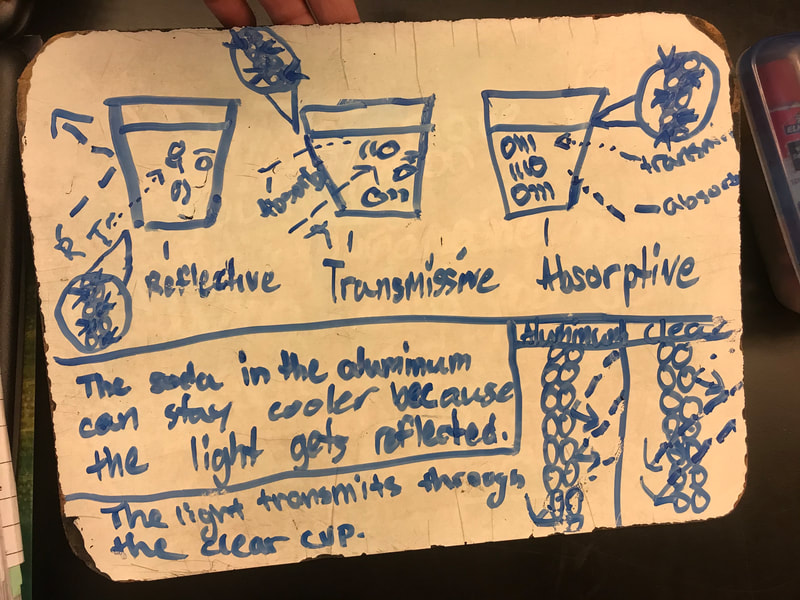
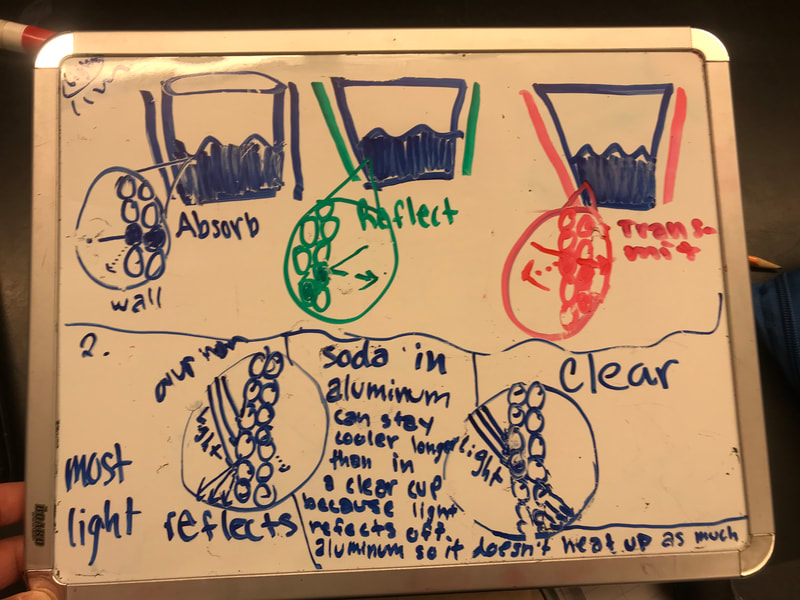
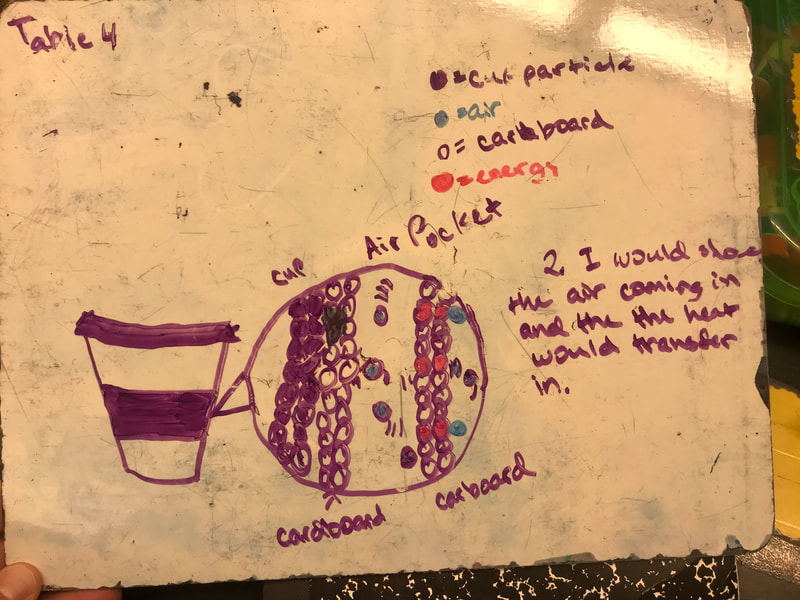
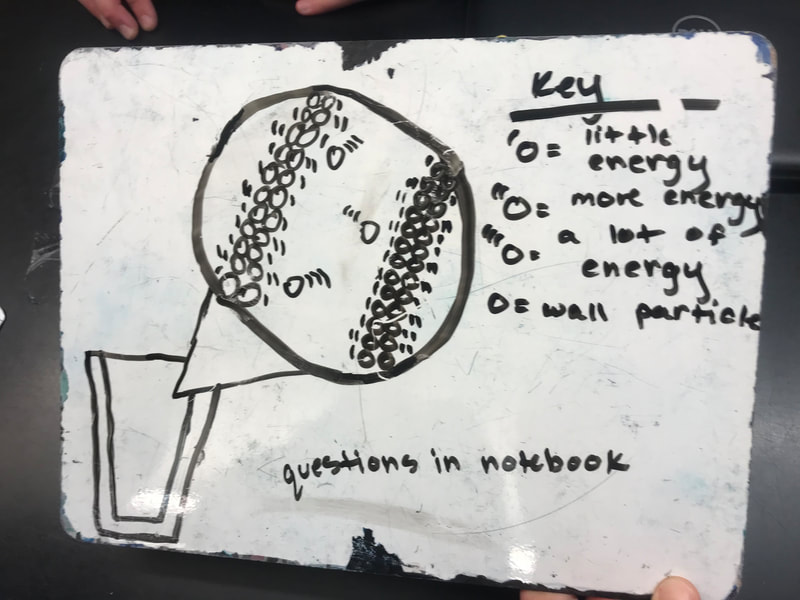
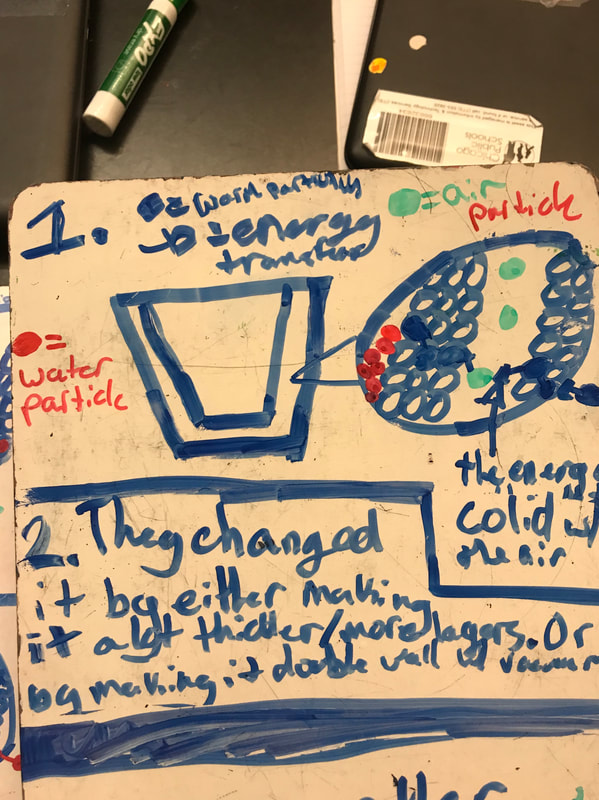
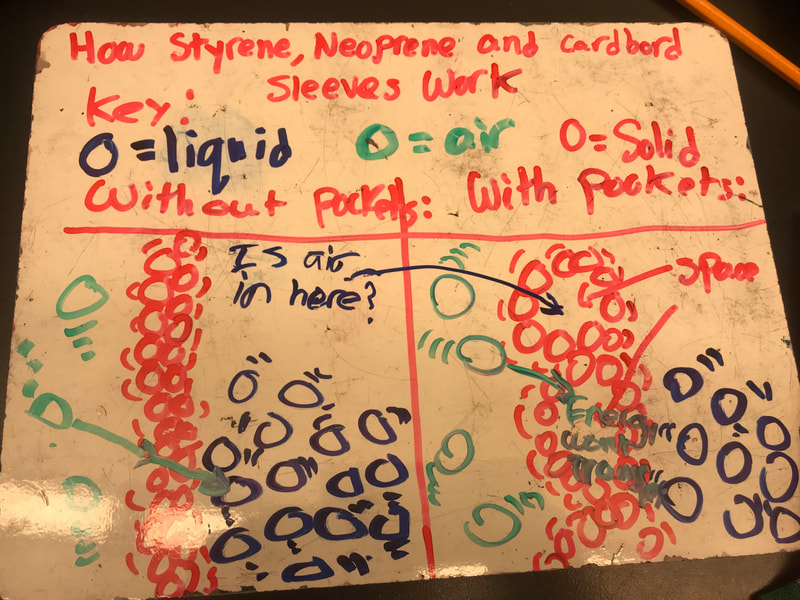
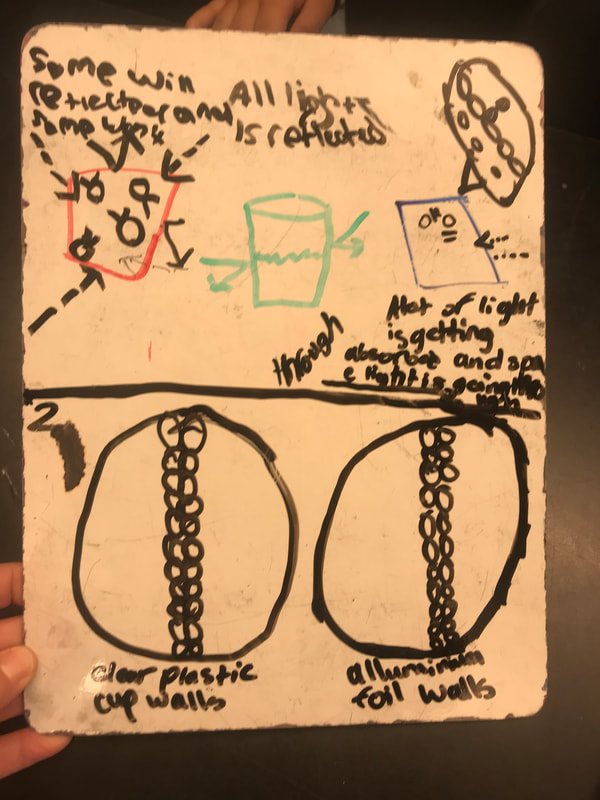
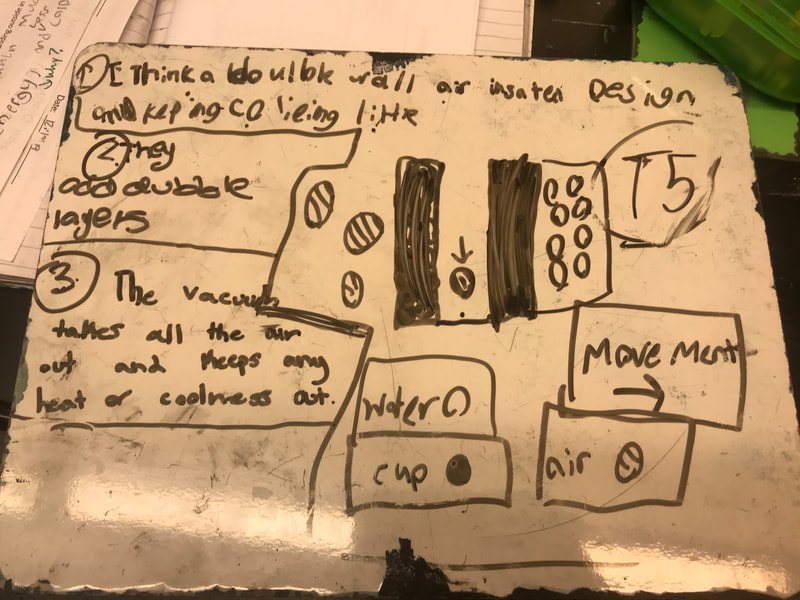
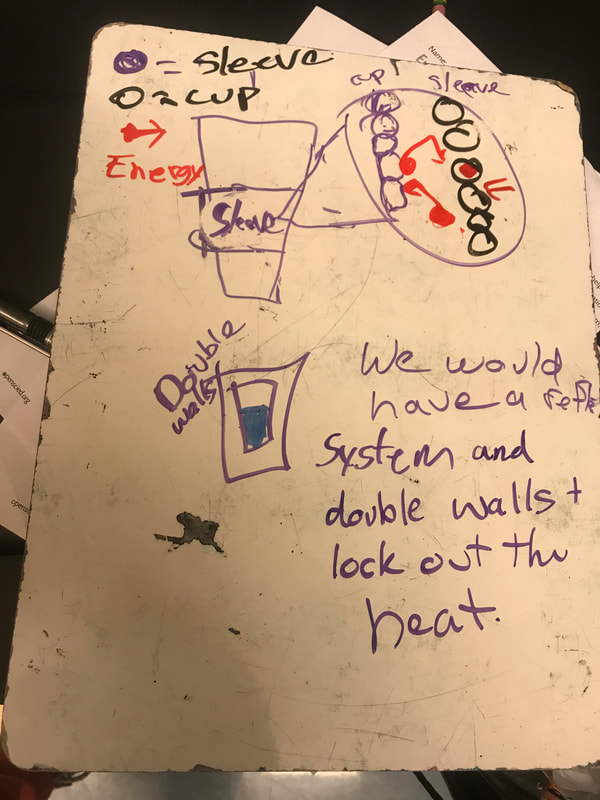
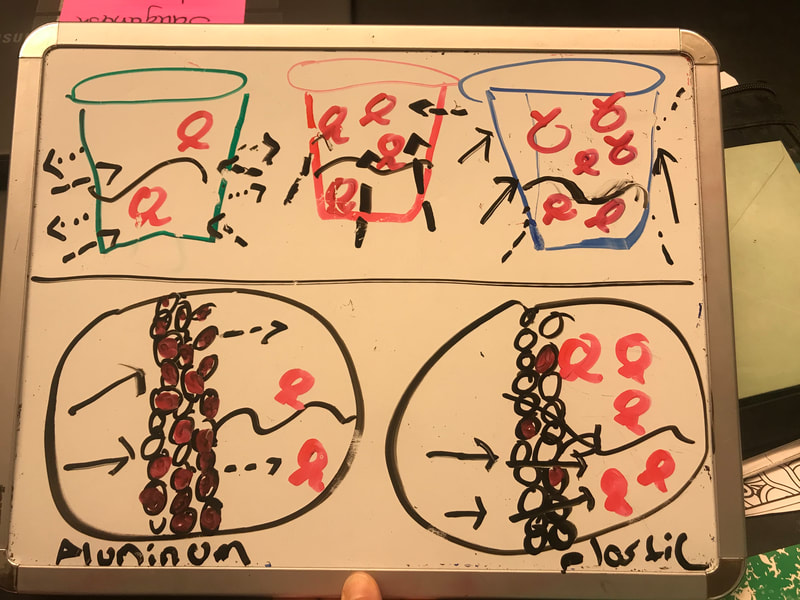
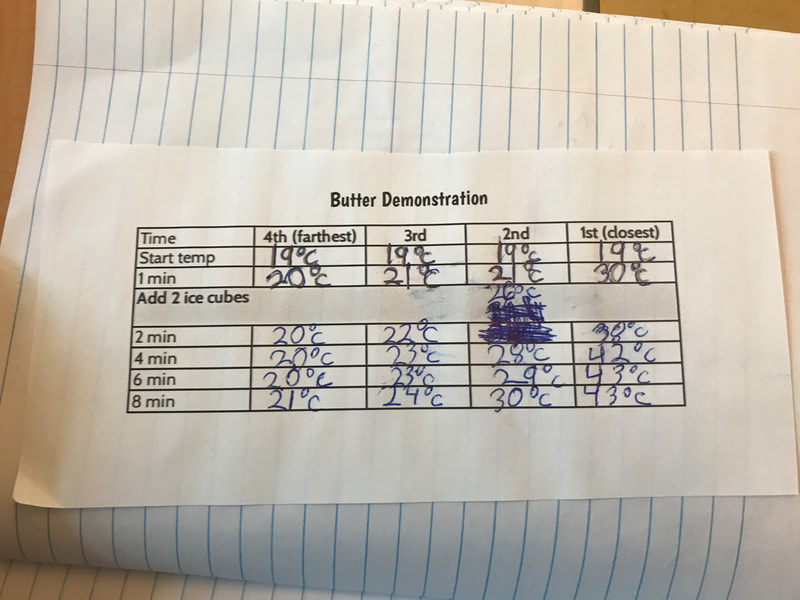
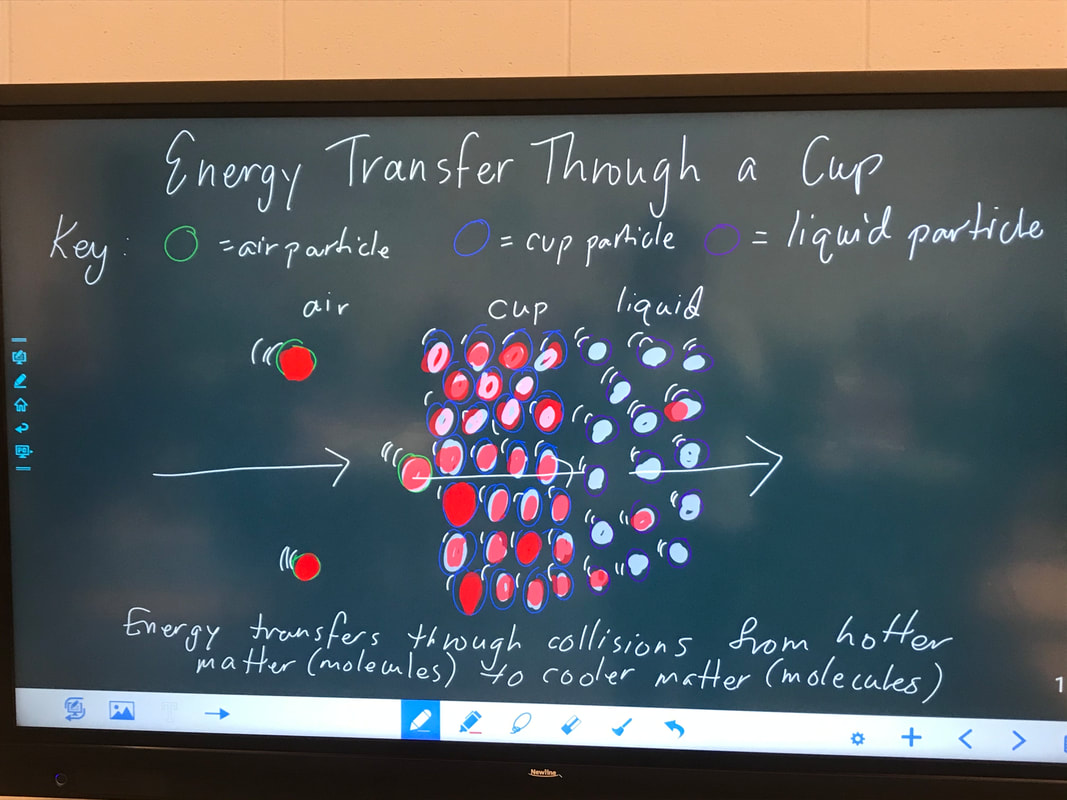
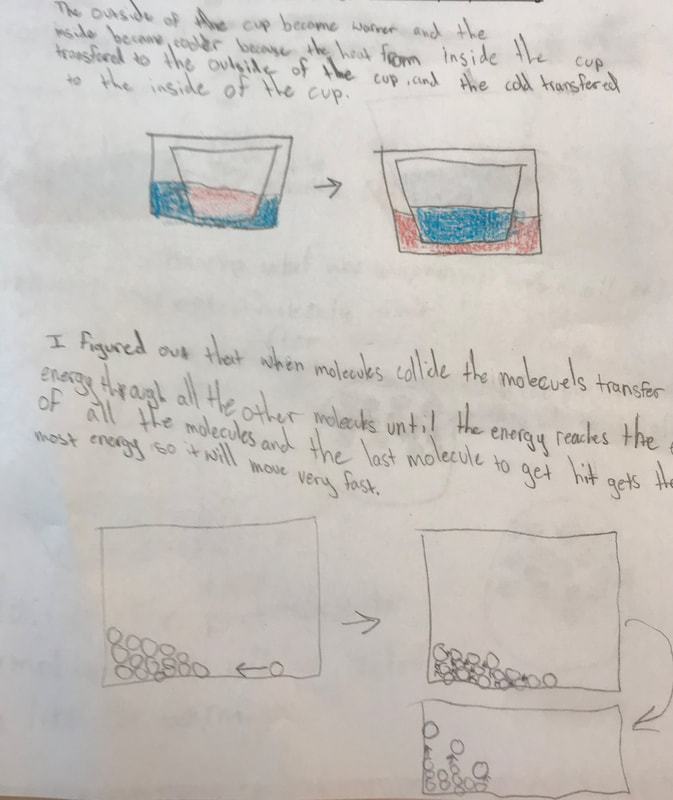
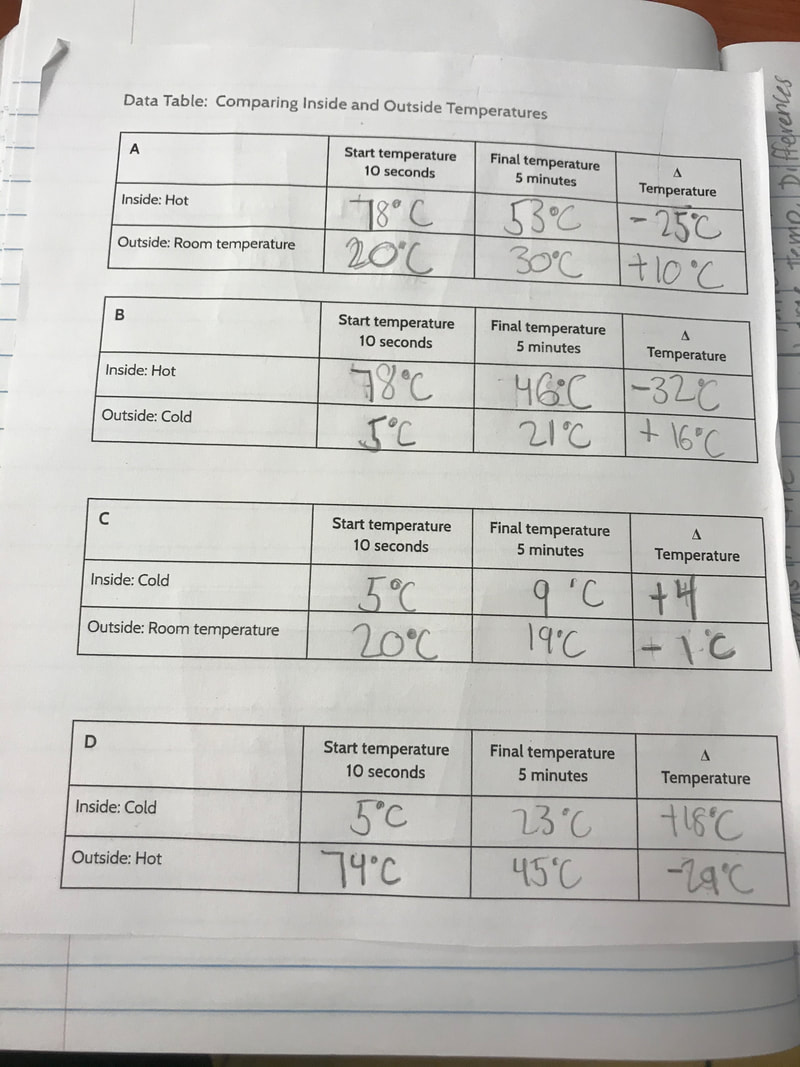
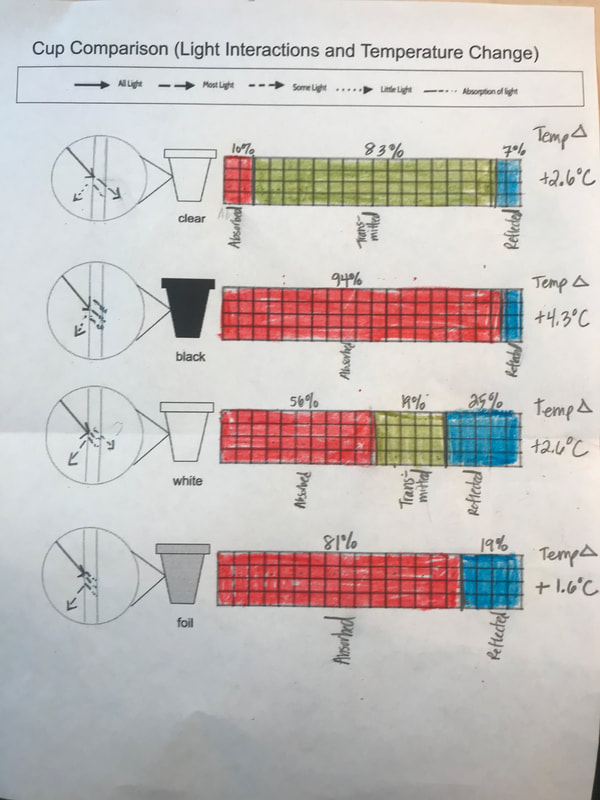
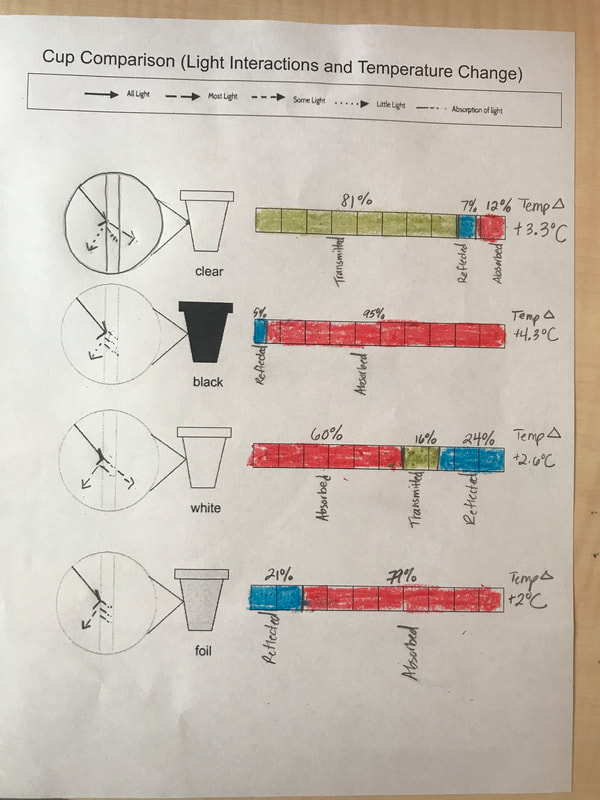
Designing, evaluating, and redesigning our cups were in full swing the last few days! After careful discussions involving lots of the science principles we've figured out, student groups were off to make a recommendation! Look at all this hard work and how it paid off! You've got one proud teacher here!
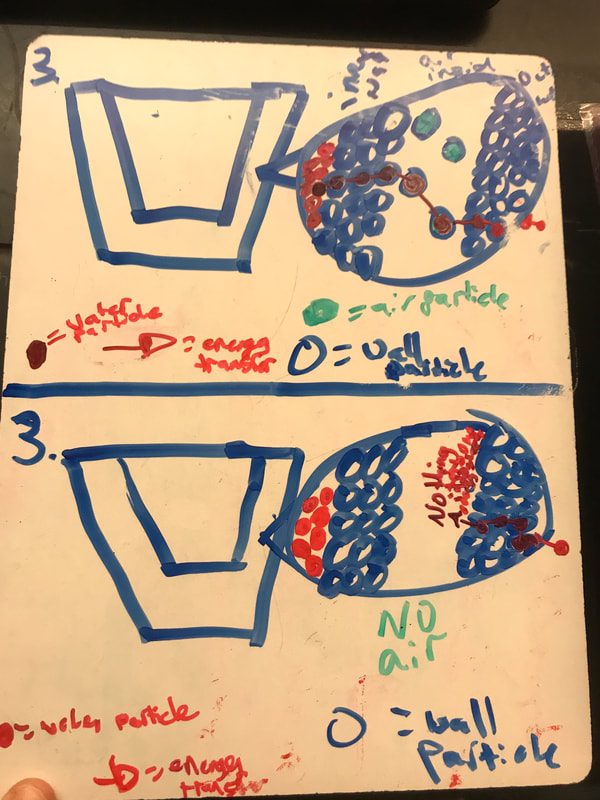
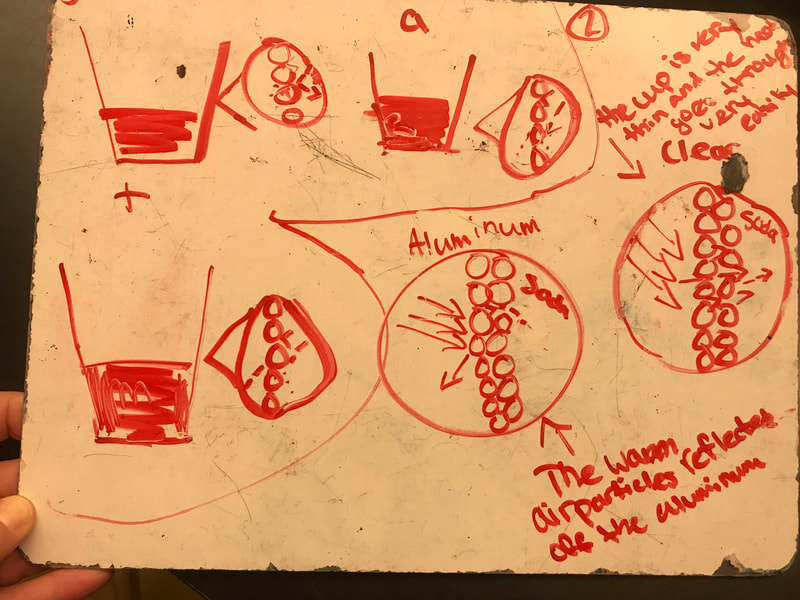
No two cups were the same, but many groups met the criteria of the challenge to minimize energy transfer all staying within the constraints they set forth!
No two cups were the same, but many groups met the criteria of the challenge to minimize energy transfer all staying within the constraints they set forth!












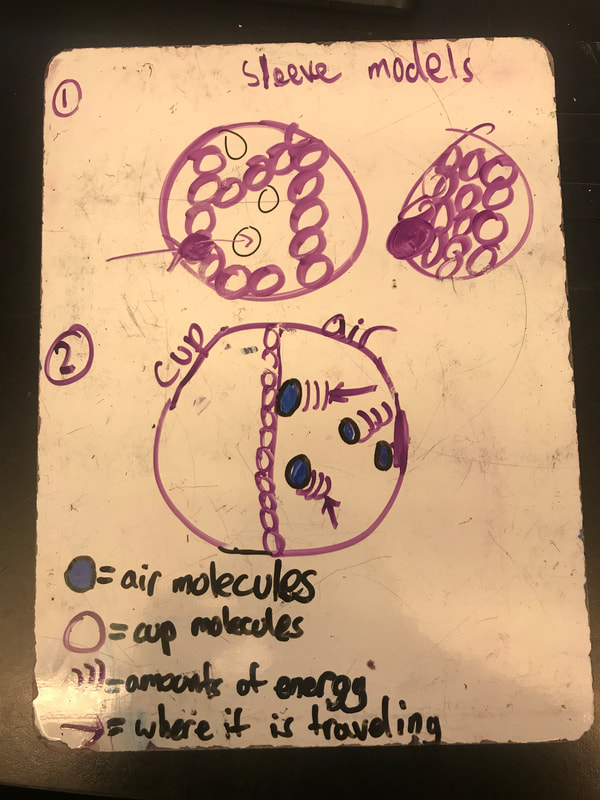
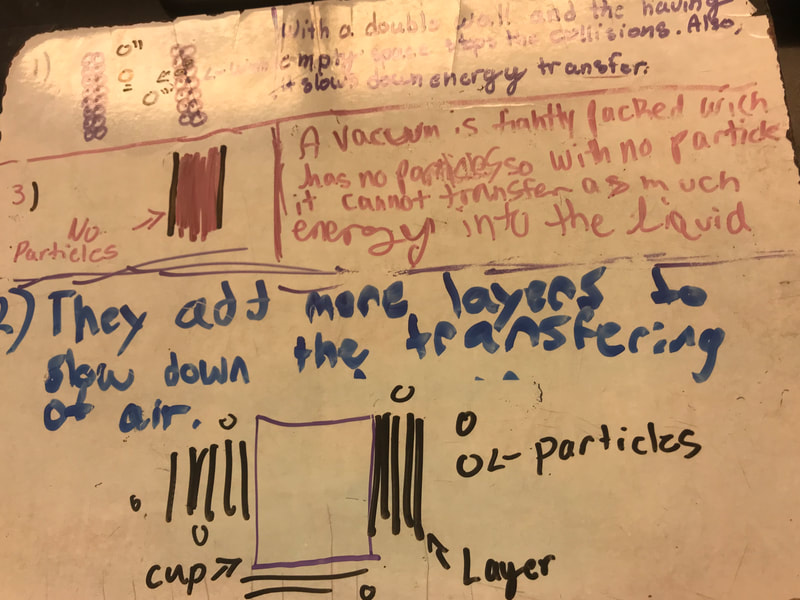












 RSS Feed
RSS Feed