Our animations were great! We're really figuring out what's happening to all these particles when they collide...
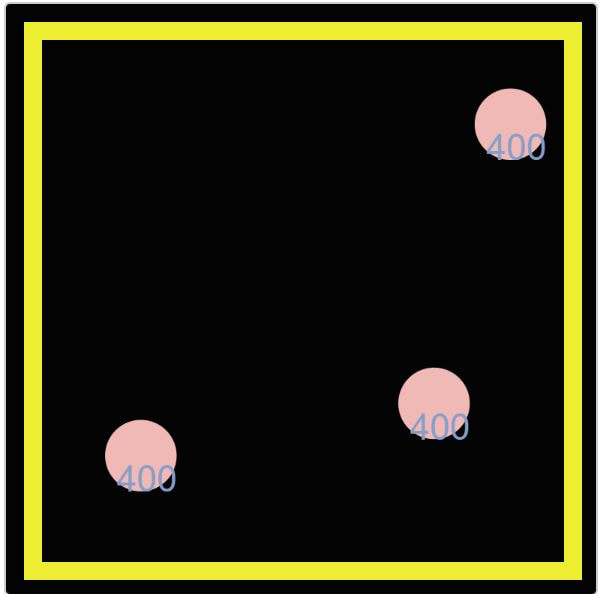
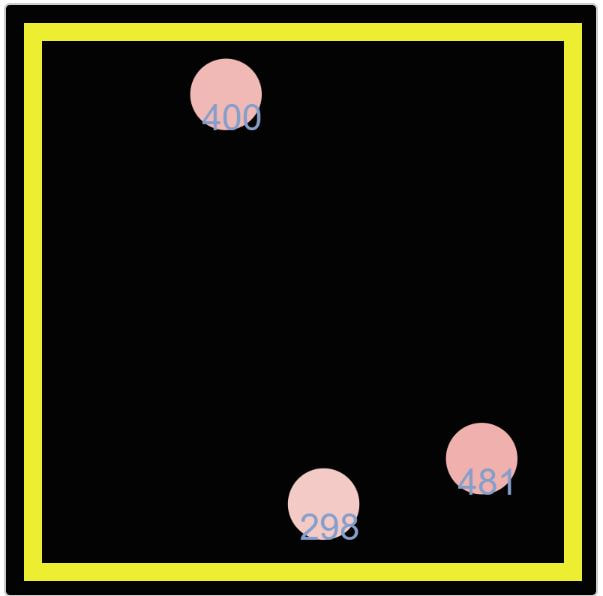
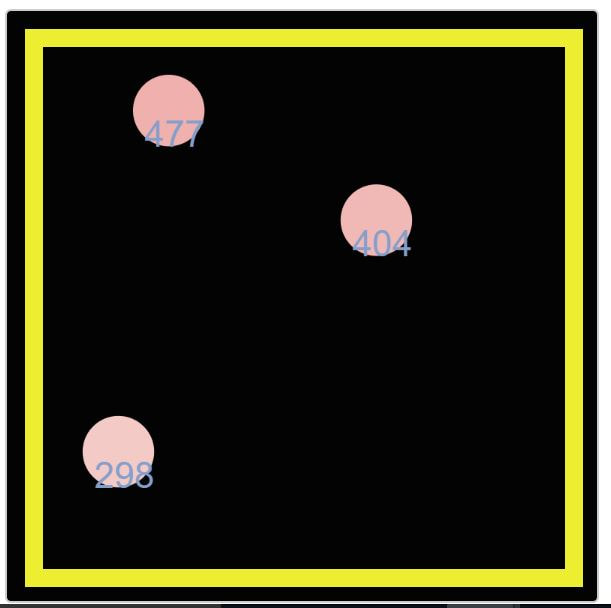
Digging a little deeper, Mrs. Brinza found another simulator that actually puts in the amounts of energy we've been figuring have been transferring. We see how the molecule with more energy transfers its energy when it collides with a molecule with less energy. And we're also seeing the kinetic energy values increase as more molecules enter the system.
Digging a little deeper, Mrs. Brinza found another simulator that actually puts in the amounts of energy we've been figuring have been transferring. We see how the molecule with more energy transfers its energy when it collides with a molecule with less energy. And we're also seeing the kinetic energy values increase as more molecules enter the system.
We're also recognizing that our cup systems have many parts, and we've figured out LOTS about the air around the cup, the cup walls themselves, and the liquid inside the cups. We're also figured out a TON about energy transfer and how each molecule (with different amounts of kinetic energy and therefore a different temperature) affects the molecules it interacts with.
Building out our cup system is getting us thinking the following:
1. The air outside the cup interacts with the walls. Since the air molecules have more energy than the solid cup molecules, they'd transfer their energy to the cups' walls.
2. From there, the cup walls would be next to the liquid molecules, colliding into them, transferring the energy to the liquid, making it have more energy, and therefore, be hotter!
BUT...what if the liquid is not cold on the inside and instead is hot on the inside? Do those molecules interact with the cups' walls and then the air? Or what? We're still trying to figure out this idea about whether it's heat that's moving or cold that's moving...
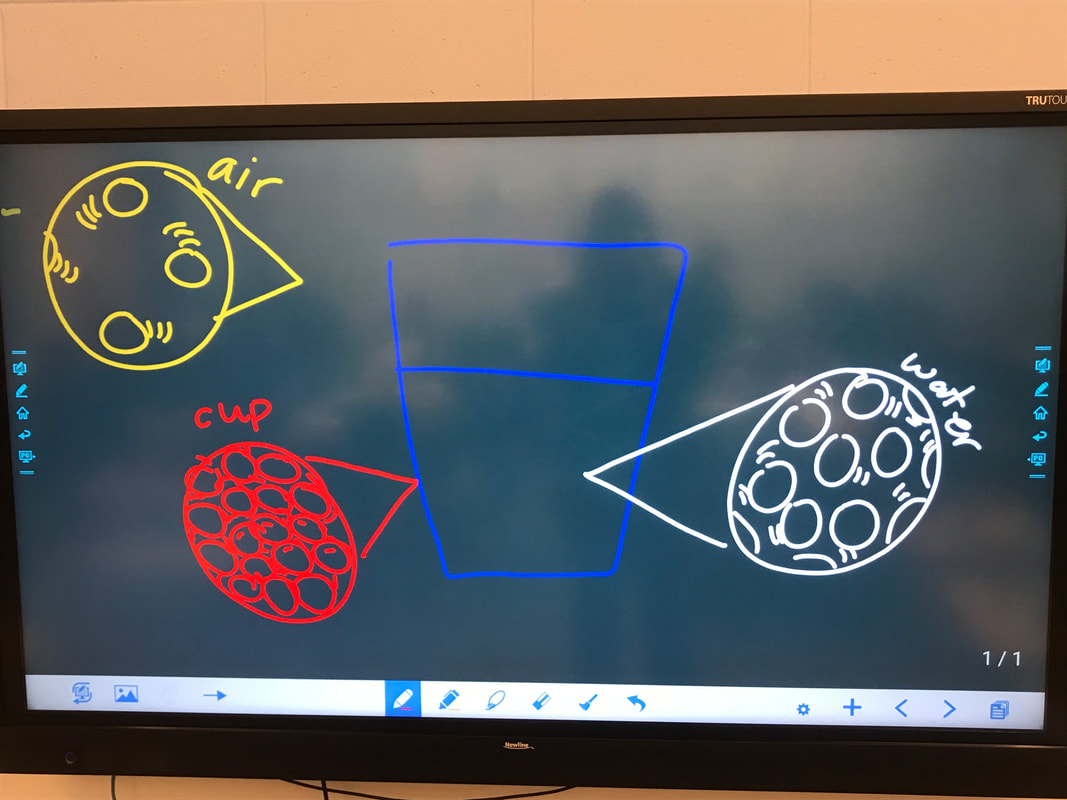
So we modeled our thinking with some marbles to represent what we know about gases, liquids, and solids (all at different temperatures with different amounts of energy) and how they interact with one another, all in hopes that this will explain how the fancy cups work better at keeping hot liquid hotter and cold liquids colder than regular 'ol cups...
1. The air outside the cup interacts with the walls. Since the air molecules have more energy than the solid cup molecules, they'd transfer their energy to the cups' walls.
2. From there, the cup walls would be next to the liquid molecules, colliding into them, transferring the energy to the liquid, making it have more energy, and therefore, be hotter!
BUT...what if the liquid is not cold on the inside and instead is hot on the inside? Do those molecules interact with the cups' walls and then the air? Or what? We're still trying to figure out this idea about whether it's heat that's moving or cold that's moving...
So we modeled our thinking with some marbles to represent what we know about gases, liquids, and solids (all at different temperatures with different amounts of energy) and how they interact with one another, all in hopes that this will explain how the fancy cups work better at keeping hot liquid hotter and cold liquids colder than regular 'ol cups...
| | | |
 RSS Feed
RSS Feed