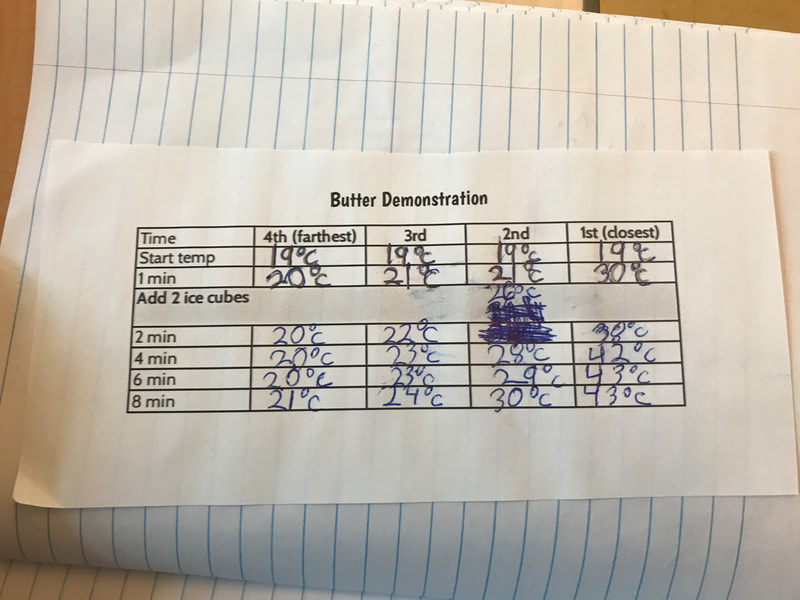
So after careful reflection, we still didn't have agreement on whether it was the heat moves or the cold moves, as we have some evidence to support ideas from both sides. But one more investigation (with butter no less) might help!
If the heat under the butter moved, then we'd see an increase in temperature over time. But if the cold moved, then we'd see a decrease in temperature over time. What's the data tell you?
If the heat under the butter moved, then we'd see an increase in temperature over time. But if the cold moved, then we'd see a decrease in temperature over time. What's the data tell you?
The molecules are colliding with more heat, transferring their energy to the molecules nearby (which have less energy). We see an increase in temperature throughout, providing more evidence that molecules with more energy collide with molecules with less energy, transferring the energy onward...
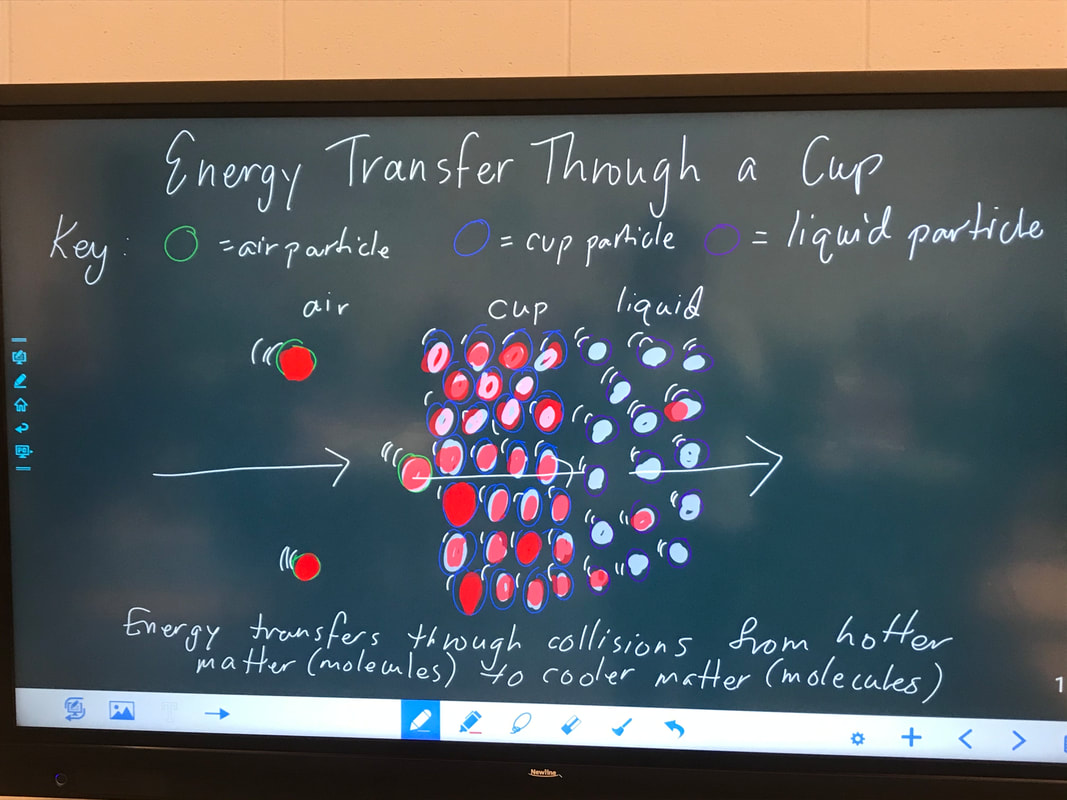
We worked towards consensus, thinking how this butter investigation could explain how collisions between air, the cup, and the liquid inside get the cold liquid to warm up. Look where we've come, folks!
We worked towards consensus, thinking how this butter investigation could explain how collisions between air, the cup, and the liquid inside get the cold liquid to warm up. Look where we've come, folks!
What should we do with all this science knowledge? Should we attempt to redesign a cup to keep a beverage cold? I say, "Why not!??!"

 RSS Feed
RSS Feed