After designing two rounds of cups, students compared their two designs and made a recommendation as to which cup should be mass-produced! We had some cup designs that didn't change in temperature at all, and some that changed A LOT! We had some expensive designs and others that ran super cheap! We also had some cups that were NOT very environmentally friendly, too! All in all, each of our groups figured out a TON about energy transfer, especially at the particle level! Way to go 6th grade!
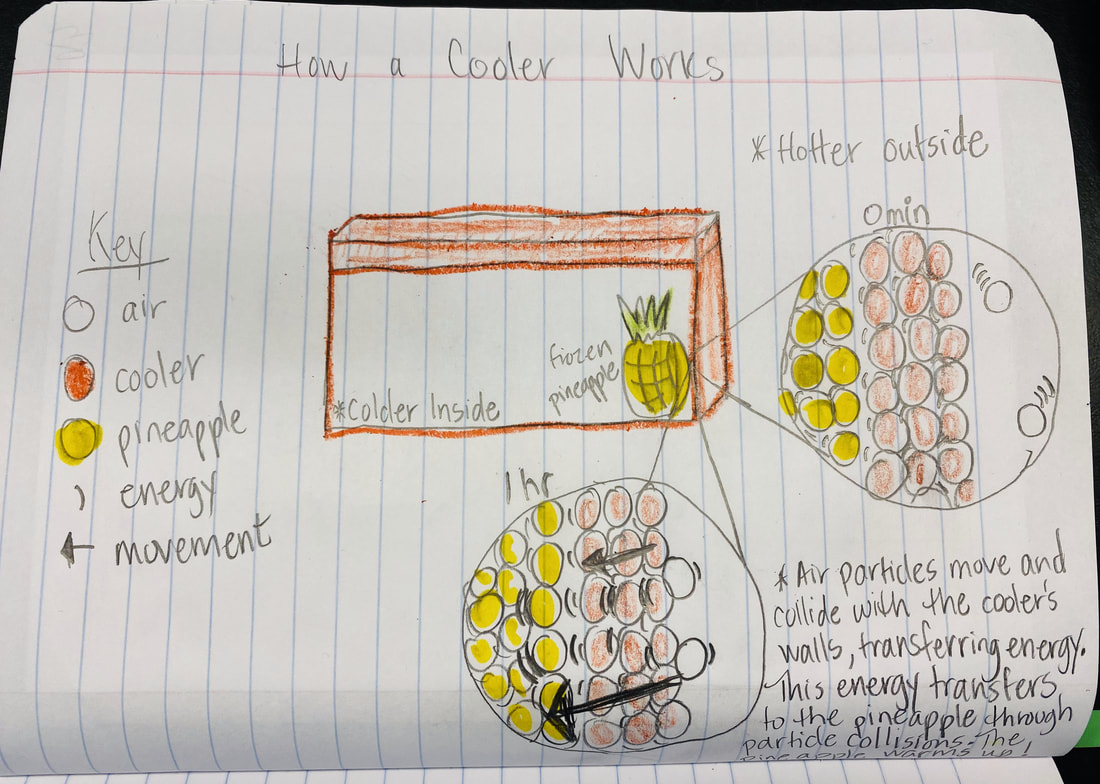
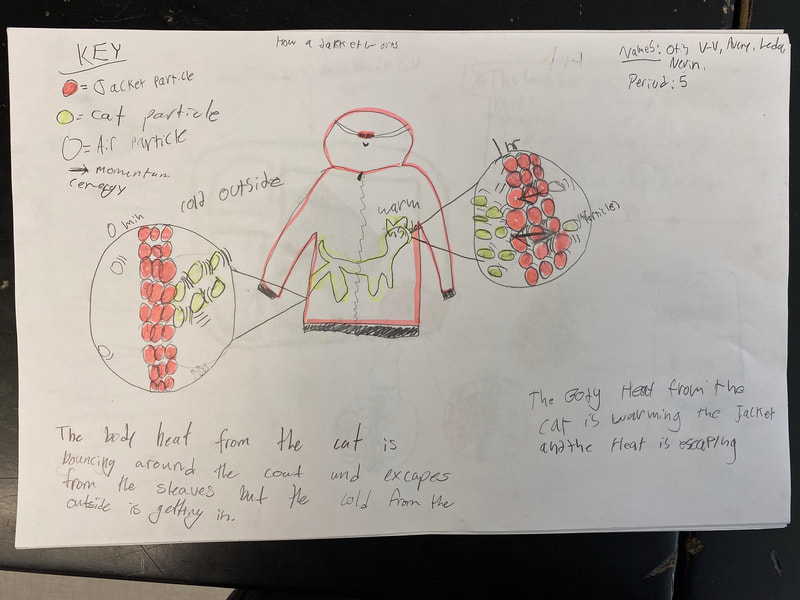
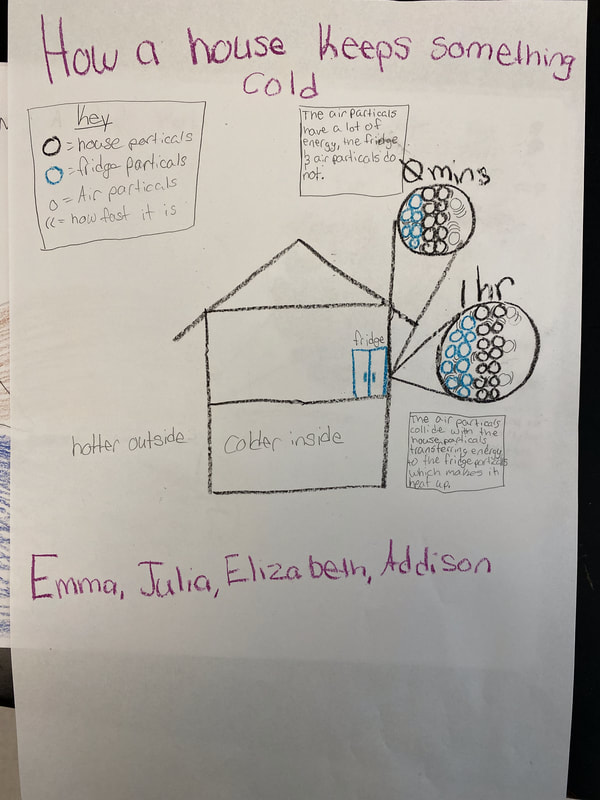
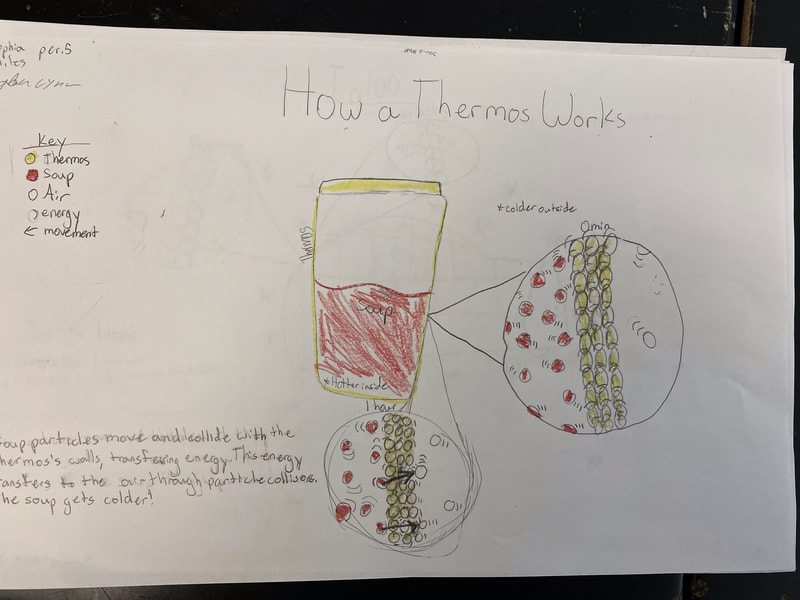
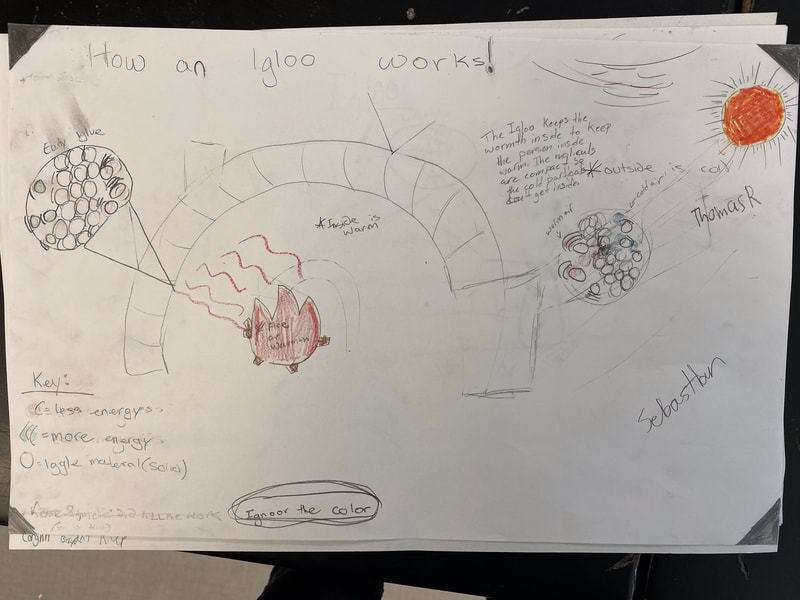
After turning to our related phenomena list from the beginning of the unit, students quickly realized that all the things that they identified that work to keep things hot or cold are just like our cup systems! Working together, we developed a model for another system that each of the classes identified: a cooler! Student groups then picked a related phenomenon and went to town developing a model to explain how it worked to keep something either hot or cold!
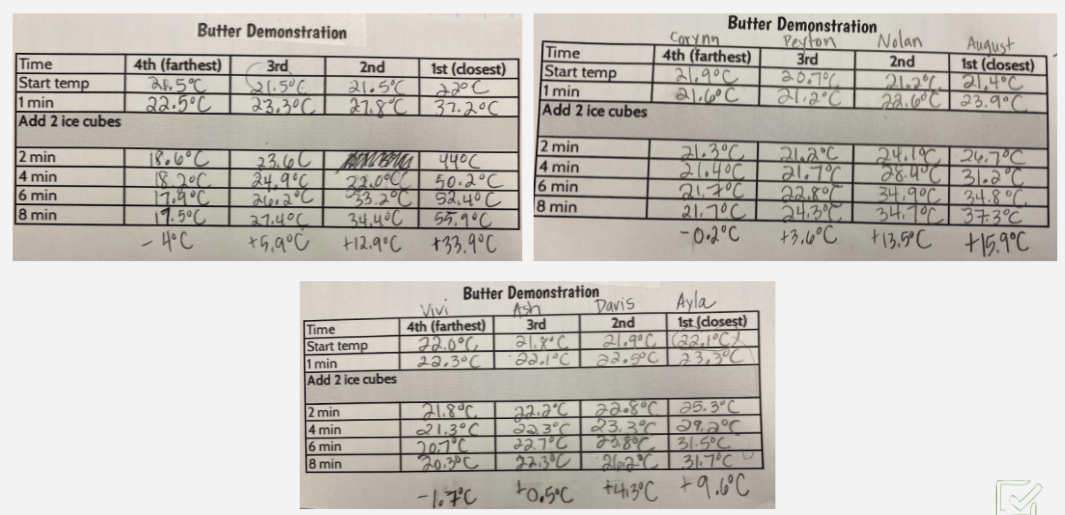
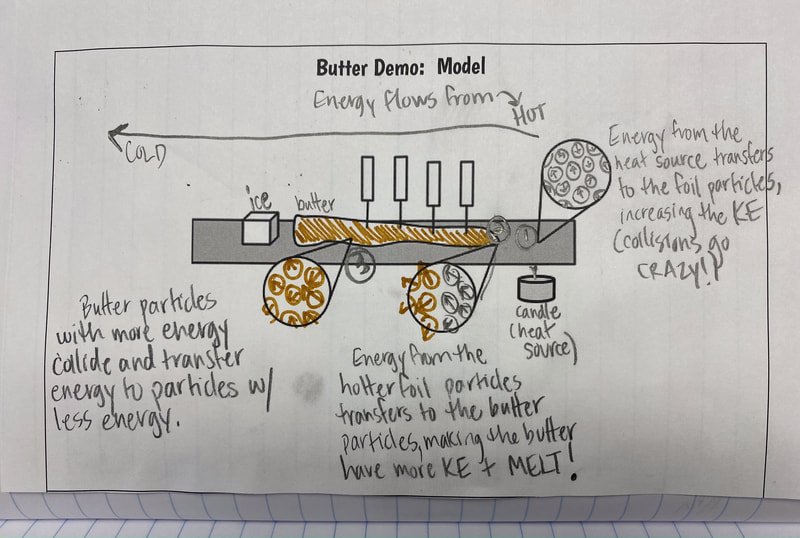
After the long Thanksgiving break, we were ready to jump back in! We reviewed what we figured out about energy transferring between states of matter, and recognized we were still a bit unsure if heat or cold moves. So we turned to something Mrs. Brinza had a surplus of over the holiday...BUTTER! With Mrs. Brinza's guidance, we were thinking that we could see if the temperature changing in butter over time both close to and far from a heat source could give us any solidifying evidence about energy transfer. Check out three class' data below. We agreed that our data helped prove what we were already thinking--that the heat moves. We developed a model to showcase our understanding, and we also considered how what was going on with the butter is just like what's going on with our cup systems! Next steps! Explain our other related phenomena!
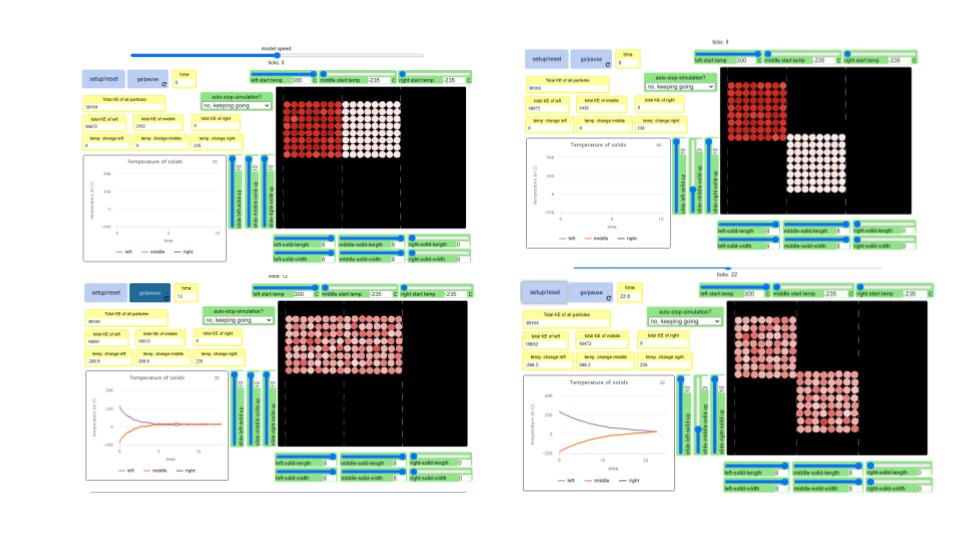
All of our use with simulators to show what particles in a gas are doing have been truly insightful! Here are some big ideas we came up with: 1. Particles with more kinetic energy transfer them to particles with less kinetic energy. 2. When you add more particles to a system, the total kinetic energy increases. 3. It seems like the temperature of a system has to be an average, as there are fast moving particles and slow moving particles with the same sample, and yet the temperature stays the same! We also began looking at a solids simulator, as the particles in a solid are really important, too! They are what holds a liquid and act as a barrier between the liquid and the air around it (oh, and we saw how gases can impact both solids and liquids)! First and foremost, we saw that like gases and liquids, solids can transfer energy, too, albeit just slower (and this makes sense since there are LOTS of particles in solids). We also saw that when one solid was bigger than another, that bigger solid seems to make more of an impact (when it's hotter, of course). And we also saw that as the area in contact increases, the energy transfer is faster. Regardless of the setup, we also saw what seems to be a reoccurring pattern: the heat transfers to the cold.
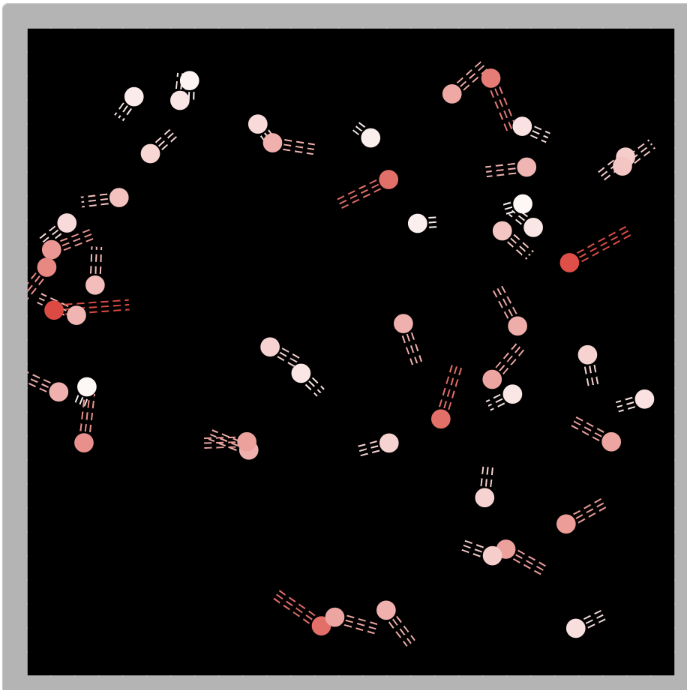
Our food coloring investigations is helping us see something we haven't considered before--that something is going on at the particle level. Using a particle simulator, we came up with the following ideas! Cold particles still move in a cold liquid--they bump into one another and are pretty close to once another. Not every particle is moving at the same speed. As you make the water warmer, the speed of the particles increases. The motion becomes faster and the space between the particles increases. The collisions get wild! We figured there's a term for the motion of particles, and this is called kinetic energy. We wanted to know even more about what's going on with the particles, so we turned to another simulator to help! Since air surround our cups, which contain liquid, our next simulator focused on the air around our cups. We came up with the following ideas using this new simulator:
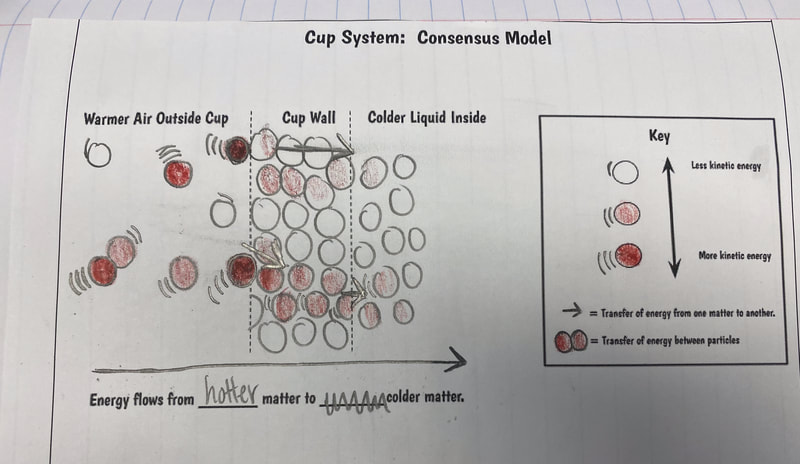
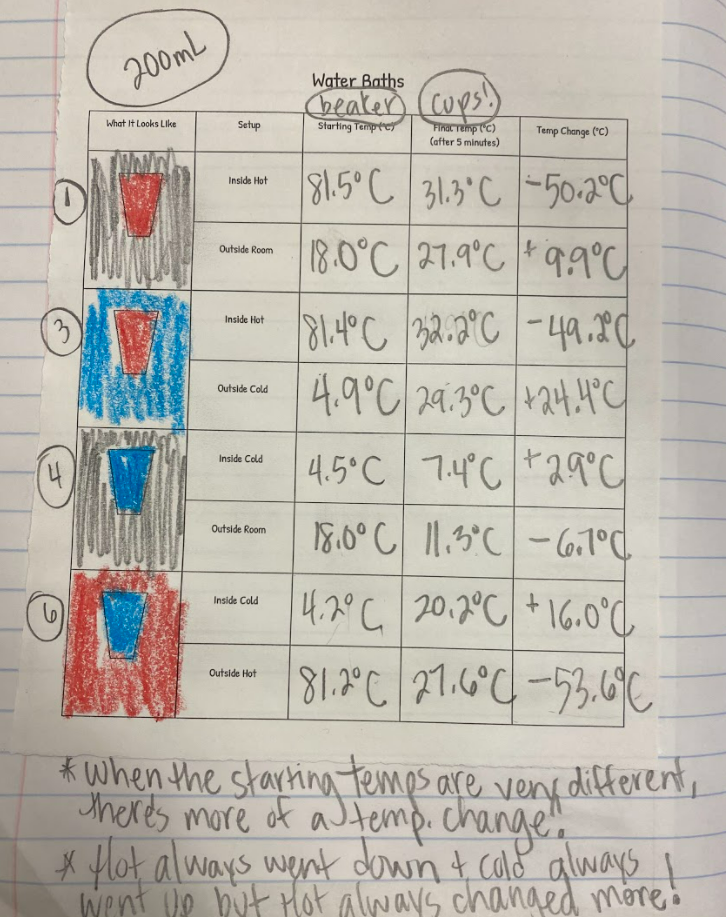
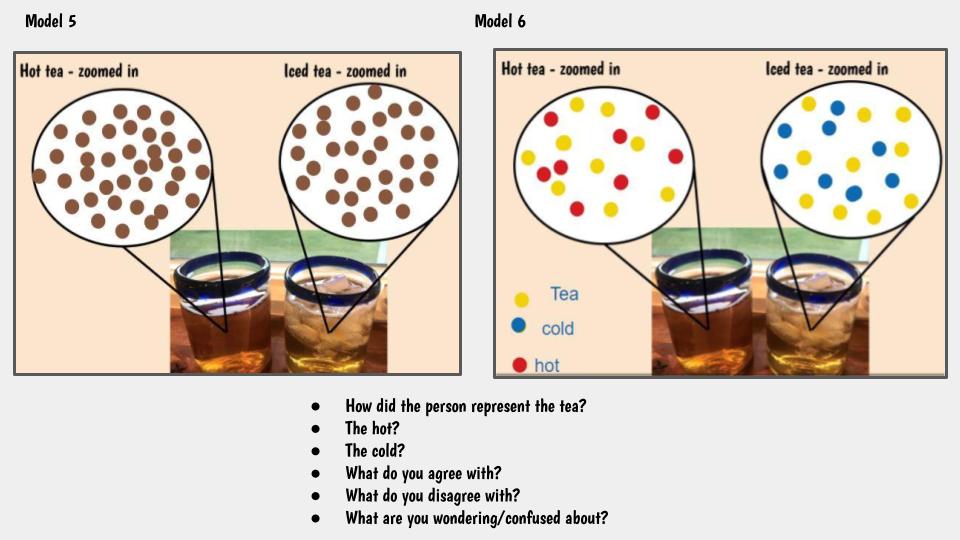
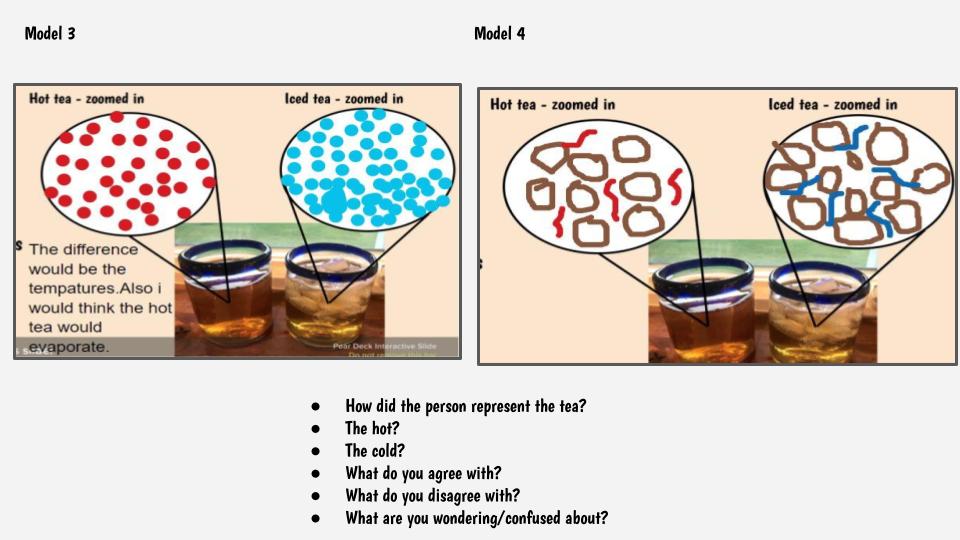
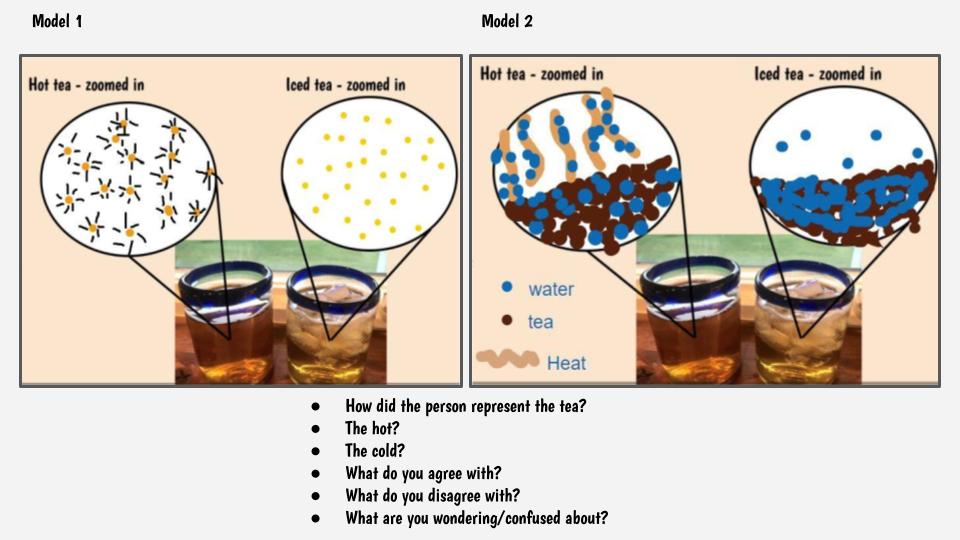
1. At a given temperature, not all particles were moving the same (some were fast and others were slow). 2. Faster particles colliding with slower particles transferred their energy. Faster particles became slower and slower particles became faster. 3. Whenever we added more particles to the simulator, the total kinetic energy increased. This made sense to us because every particle carries energy with it, so when you increase the number of particles, you inadvertently increase the kinetic energy, too! 4. You could also increase the temperature, yet keep the number of particles the same. This also increased the kinetic energy! We agreed that changing the simulator (aka "exploring" it) was fun, but challenging to narrow down any other ideas we might be missing! Tomorrow we agreed to run it in a more controlled way, which can hopefully help us consider some of these "colliding" ideas we're trying to figure out! Considering the fact that we think what's around the cups changes the cups, we decided we really wanted to think about changing the air temp around the cups. This seemed like a pretty daunting task since whenever we measured air temperature in our classroom, we got all kinds of differences. We agreed that water surrounding cups (like a bath or hot tub) could give the same feeling like a sauna around a cup! We agreed to change temperatures inside and outside the cup, and record temperatures both at the start and end of a five-minute period. This got us thinking that if hot things always cool down, and cool things always warm up, something has to be getting these changes to happen. We began thinking that there has to be a difference between hot and cold liquids at the particle level, so we considered both hot and cold tea! Students were asked to model the differences and we discussed what we agreed and disagreed with, including areas we still had questions. We turned to an investigation idea about maybe seeing the differences ourselves, and while just looking at hot, room, and cold water wouldn't cut it, adding food coloring would! So wait. Our cold cups warmed up. And our warm cups cooled down. Hot liquids move faster than cold liquids. So what's going on with all the particles to show that warmer things have more motion and cooler things have less? Is there a way to actually "see" this, or would a computer based model help?
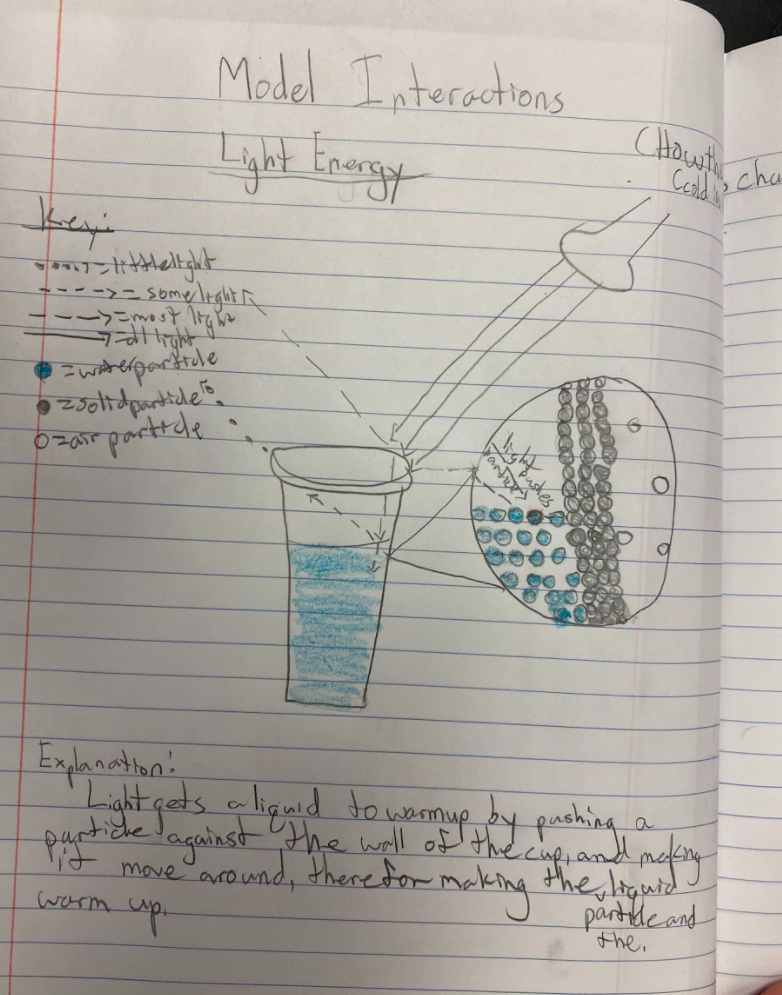
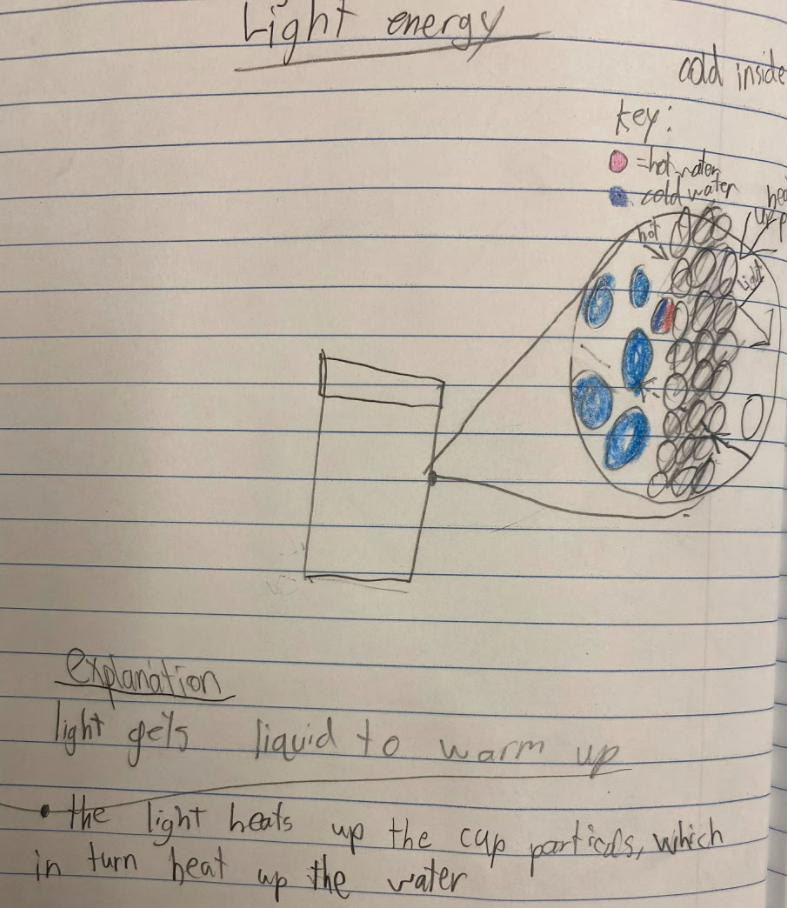
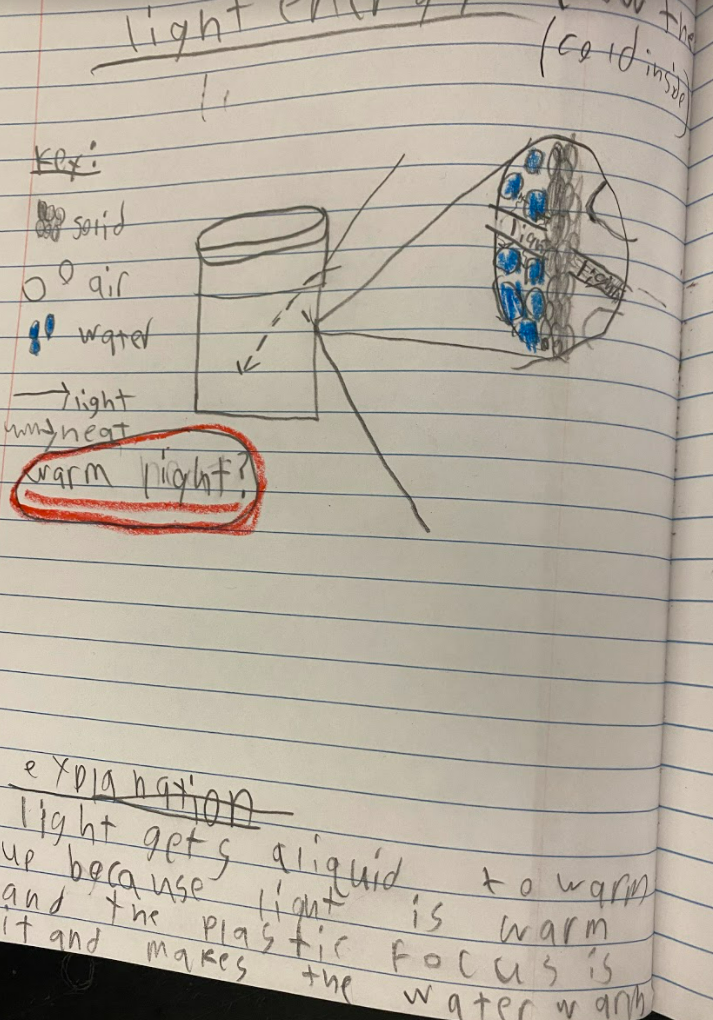
As students are trying to figure out what's going on in their cup systems now that we've got a handle on particles, their initial models are telling them a lot! We've got some great ideas on the table! After thinking about light and heat differently, including what they could be doing to particles, we began running a bunch of tests, trying to isolate heat vs. light! Our results were fairly interesting. All cups, regardless of their location, increased in temperature! Cups that were in the light increased more, and cups that reflected more light didn't increase as much. Cups that transmitted and absorbed light increased more!
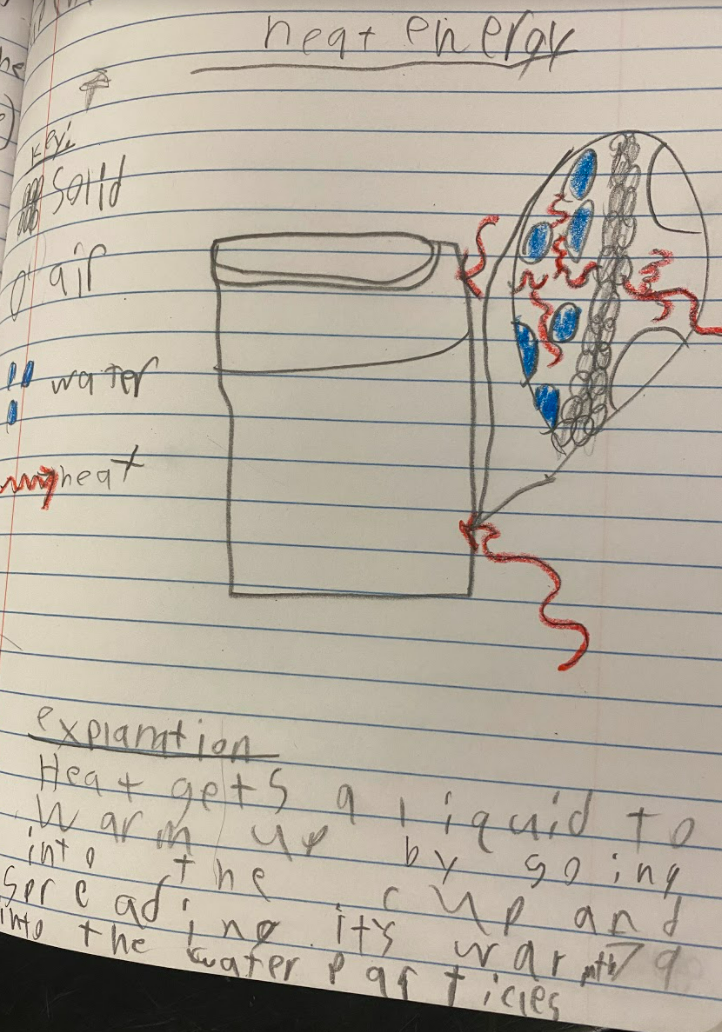
All this data collection made us think about what's really going on with the air around the cups, because when we have heat (which we think is in the air), the cups change significantly. But how can we get the air temperature around the cups to stay fairly constant?
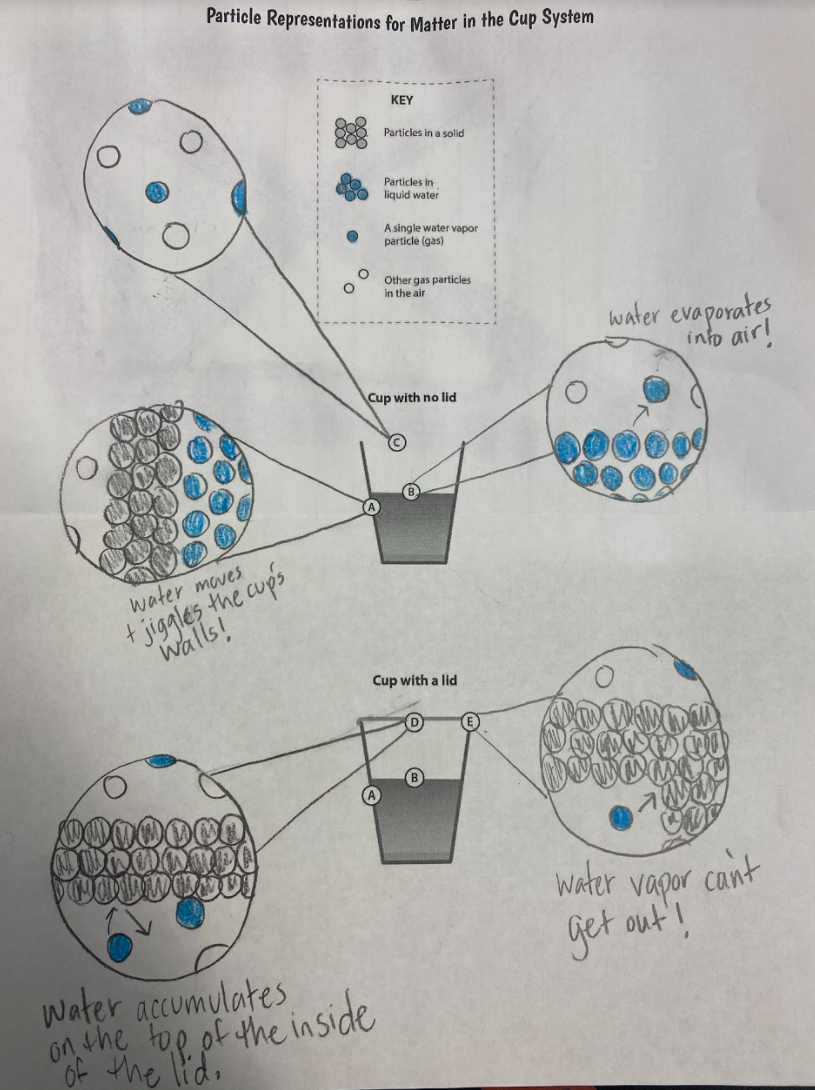
Boy did we have fun, seeing how the glass marbles (water particles) were behaving when they tried to evaporate out of the cup and hit the lid. Check out the video (and turn it on slow-mo)! We saw lots of "wiggling and jiggling" as students put it! We summarized our thinking into the following representation below! So if cups without lids lose water particles to evaporation, it's clear to us that this is why a cup loses mass. Duh, right? You lose a particle, you lose weight. However, what's odd to us is that cups that have lids on them don't really lose weight, but still lose/gain temperature (depending on whether it's hot or cold to start with).
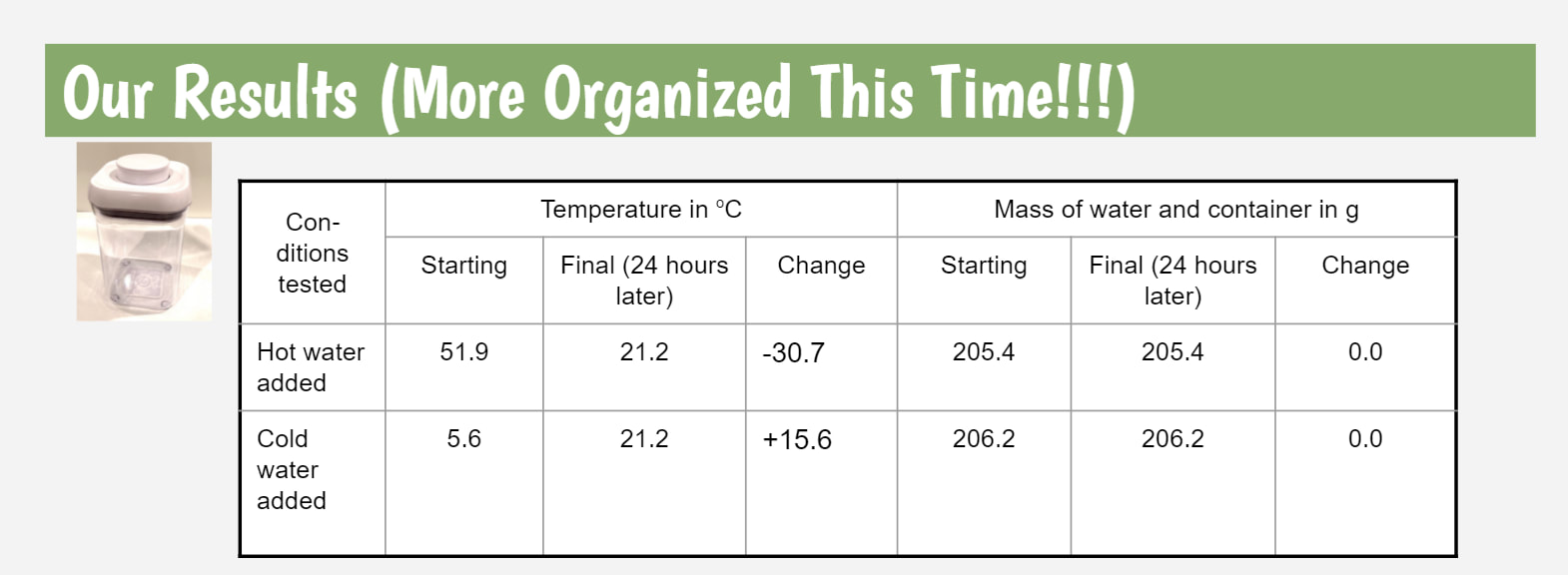
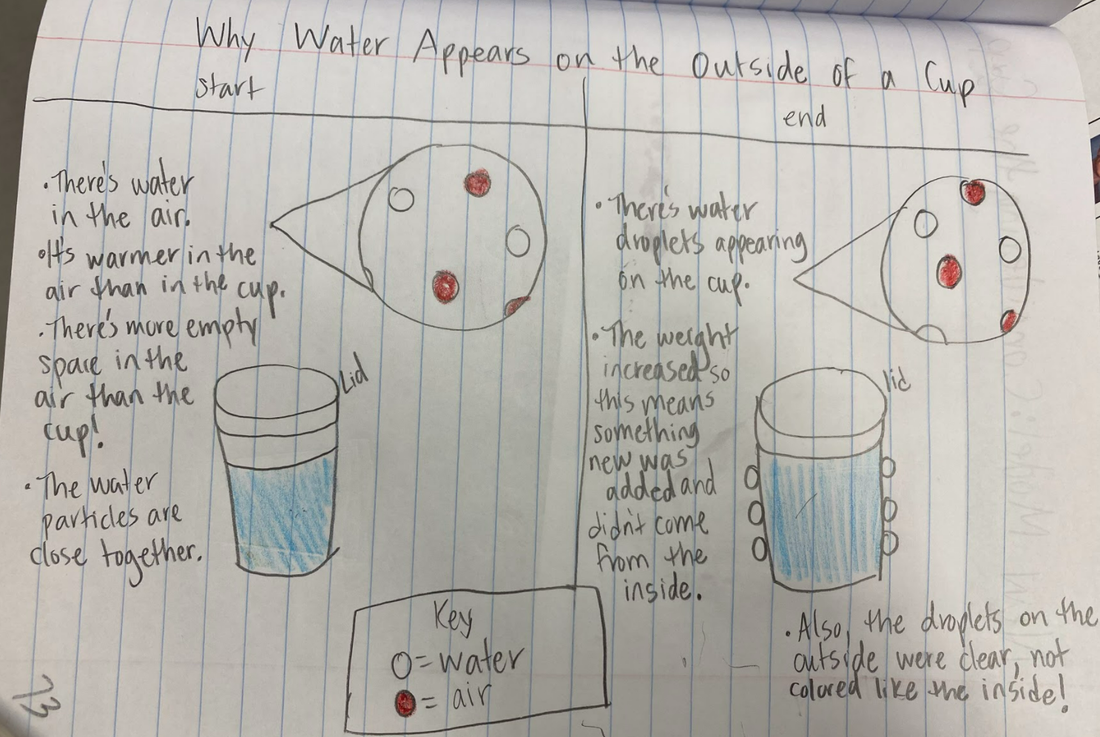
Mrs. Brinza and Mr. Carmalt first heard students' interests a few weeks ago when we began running cup tests. So here's the data from behind-the-scenes "stuff." It seems like no matter what, when it's a closed system, we're not losing weight, but we're losing temperature. So what's really going on that gets this to happen?!?!?! Is it light? Heat? Both? What are those particles really doing?!?! After seeing that our cups lost weight when water evaporated on them, we knew that water had to be in the air! So where exactly does the water come from? After a long discussion, we came to the following agreement based on data we had collected: We're also starting to see that this water in the air landing on the cup, or water ending up inside the cups walls must be related to temperature. It seems like water has to be in the air, and then it's got to be wayyyy cooler to see it back again as a droplet.
All this is getting us thinking about our cups and these little particles. What exactly are all these particles doing? How is the cup structured? |
Mrs. BrinzaSo what is it that keeps things cold or hot? Archives
December 2021
Categories |























 RSS Feed
RSS Feed