It's always a great day when we summarize what we've figured out by answering all the questions we asked to launch our unit and at other points during our unit!
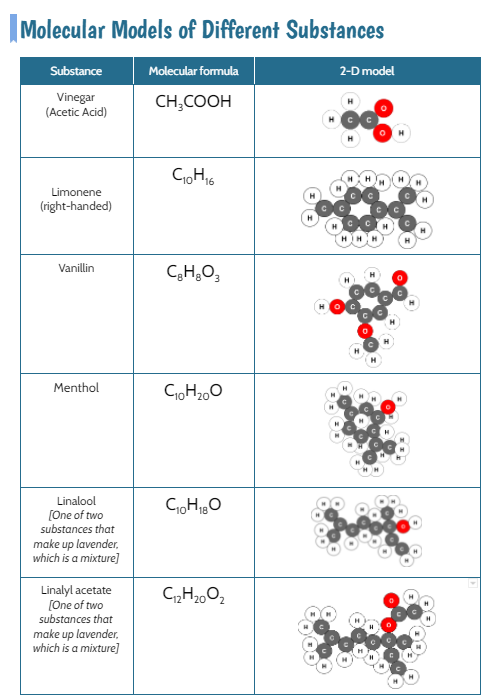
We also are wondering how the odors in bath bombs work. Like what are they? How do they reach our nose? We explored various scents, trying to guess them along with analyzing their molecular structure! We are starting to see some patterns, and with some further research, we came to the following agreements:
1. Odors are evaporated molecules that reach our nose, and receptors interpret the molecules with our brain's help! 2. Every odor is a different molecule, so odor must be a property. 3. All odors are comprised of different amounts of arrangements of carbon, hydrogen, and oxygen. 4. If one atom from an odor molecule is dropped or added, we end up with a new molecule, and therefore a different substance and odor. That explains why perfectly cooked popcorn smells different than burnt popcorn! Next steps, return to our DQB to answer all the questions we set out to ask when we launched our unit! So if not everything creates a new substance like in a chemical reaction, what happens in other processes like when water boils or when salt dissolves? If these don't make new substances, what happens? We used water molecules as an example to compare the difference between different types of processes we've seen happen! We'll be working on some final unit wrap-ups next week, as we've still got questions on our DQB about other substances in the bath bomb and how some bath bombs smell and others don't!
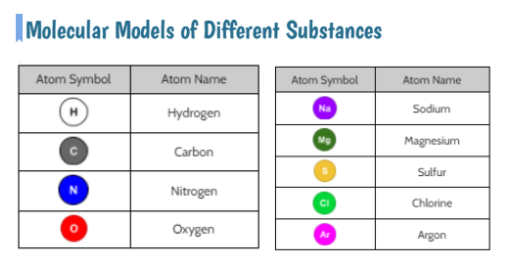
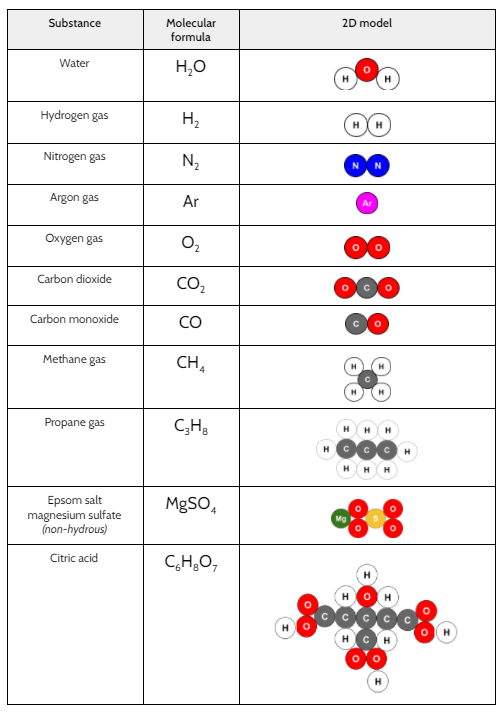
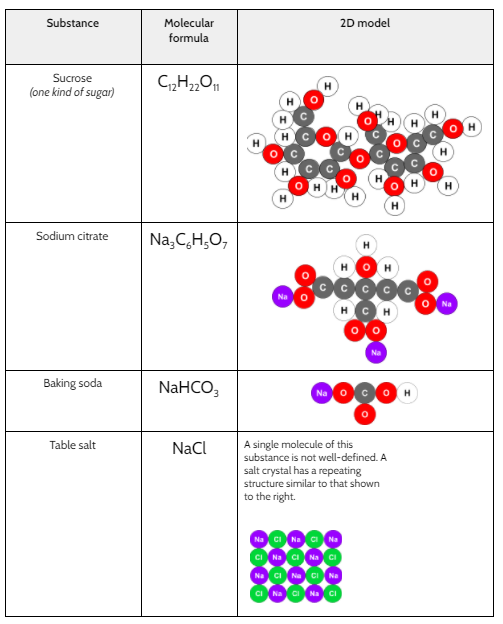
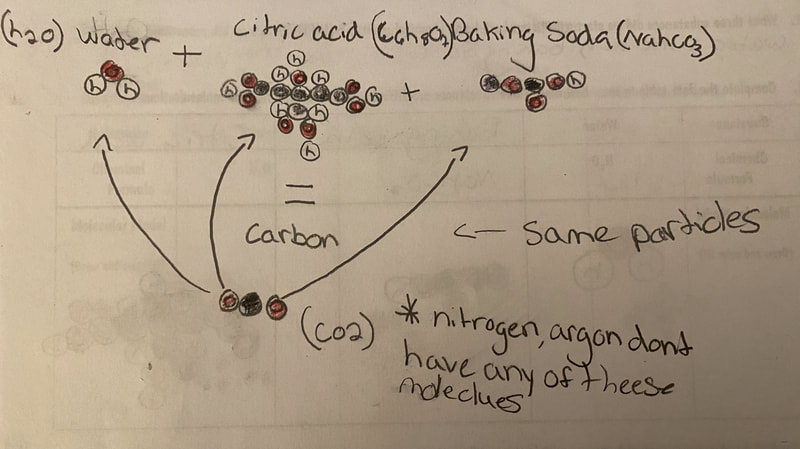
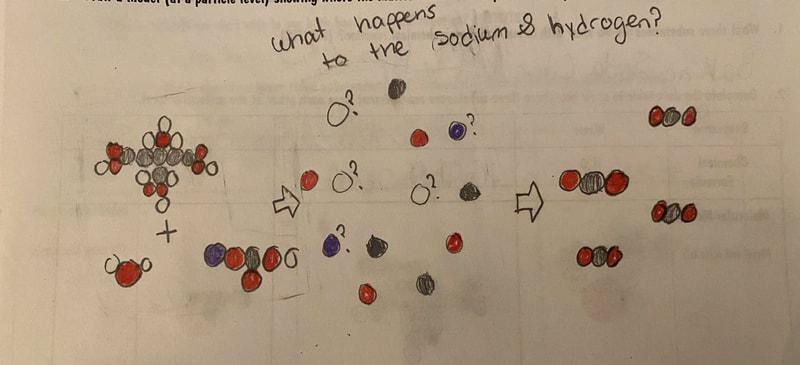
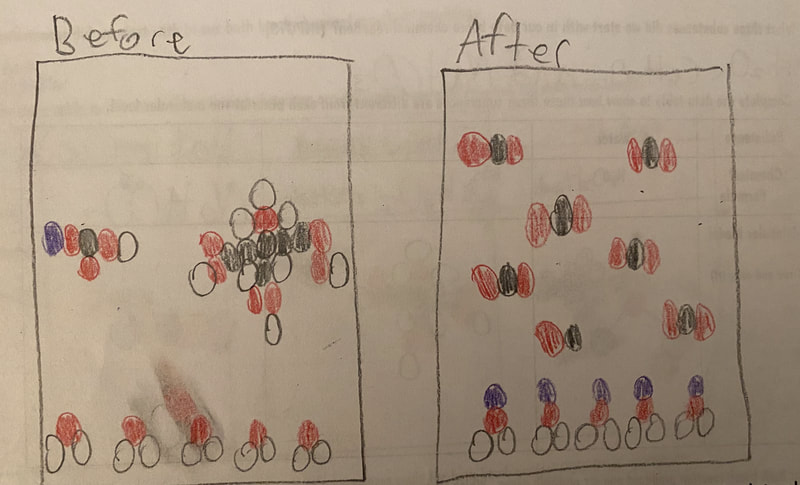
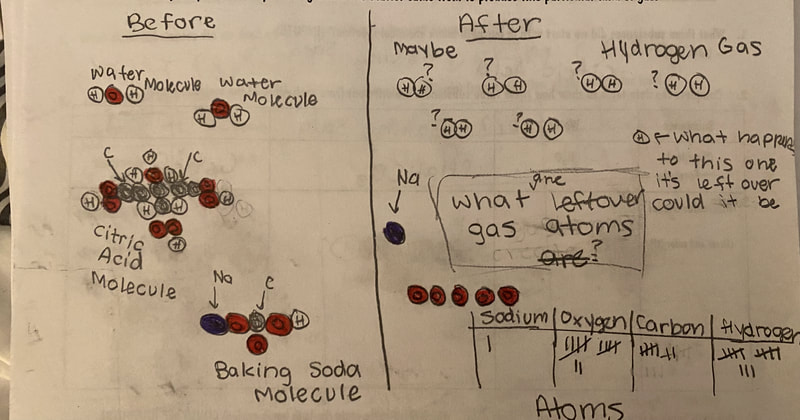
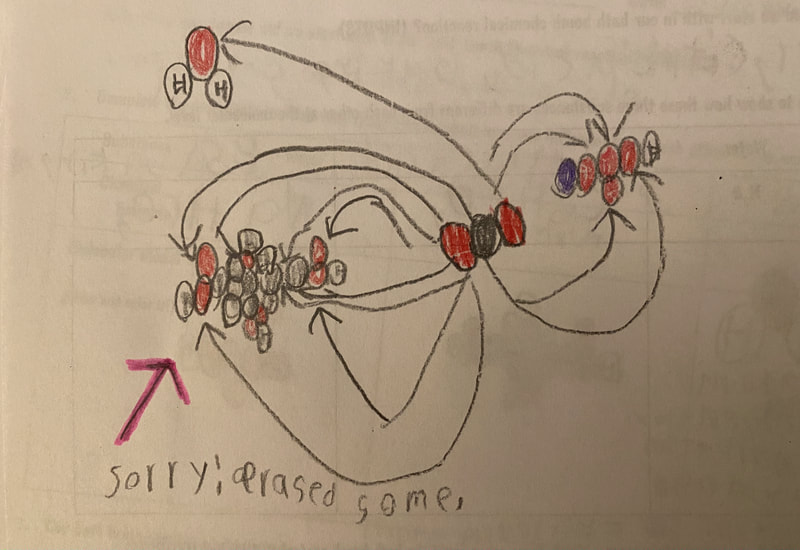
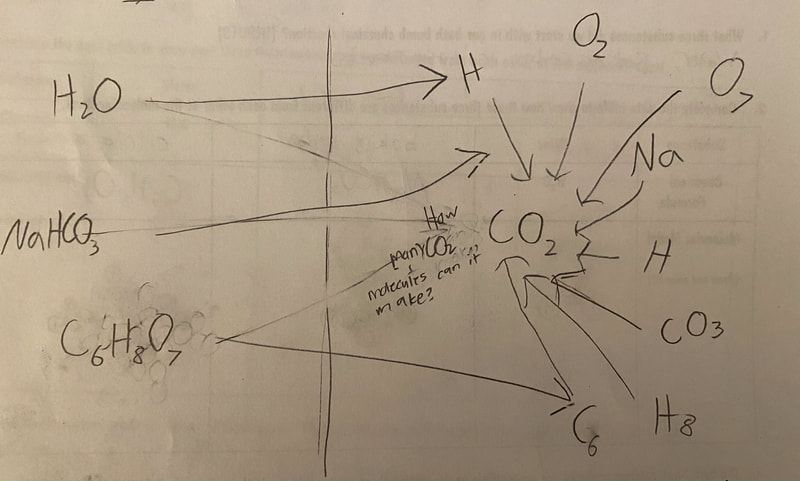
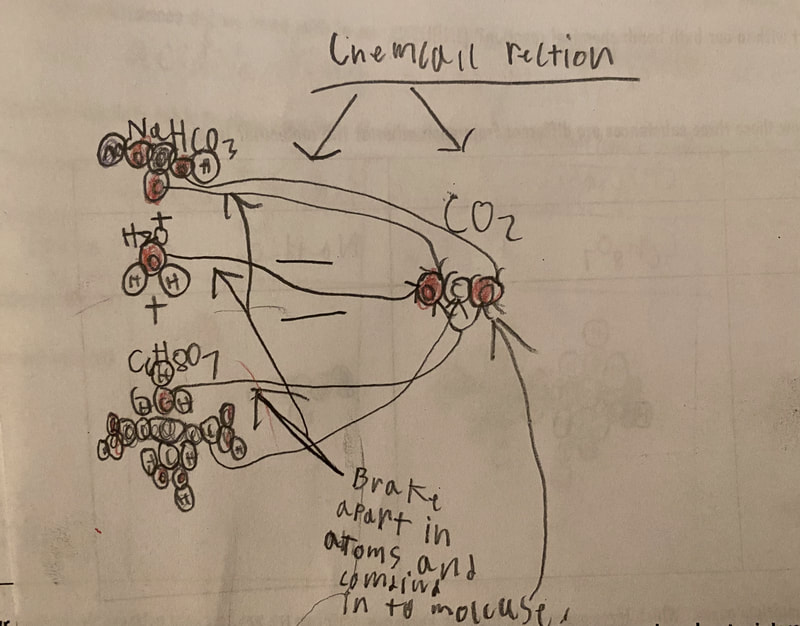
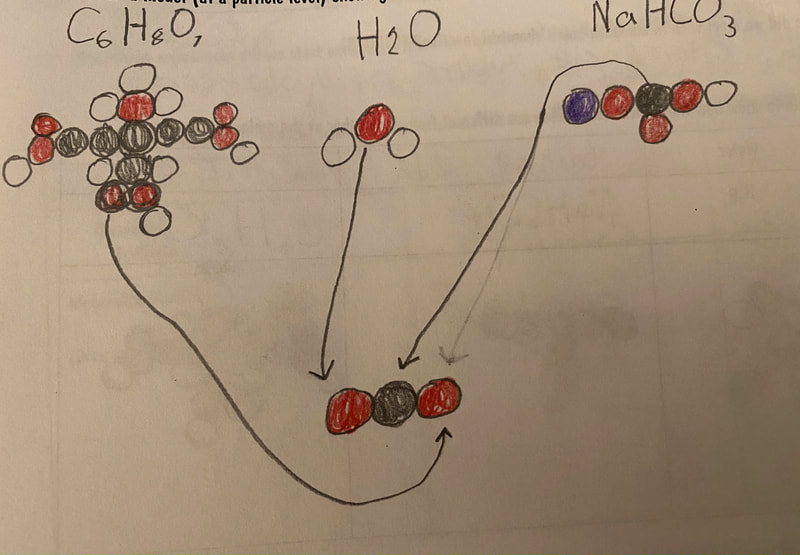
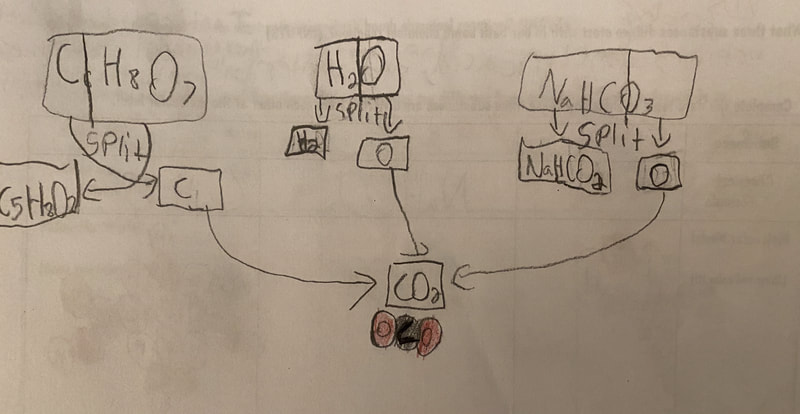
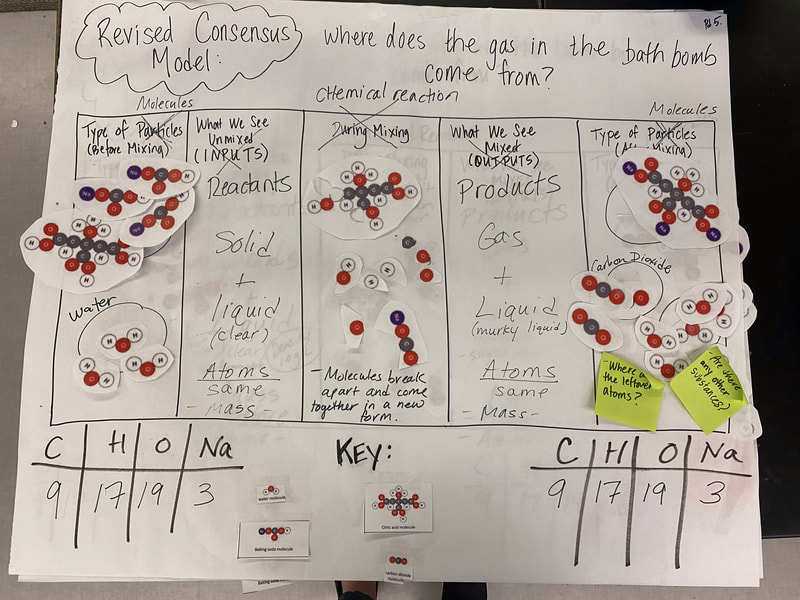
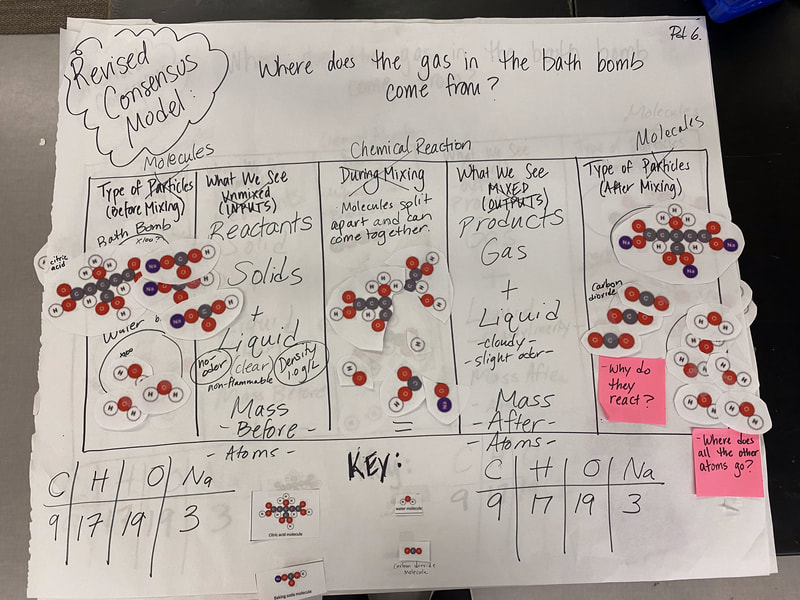
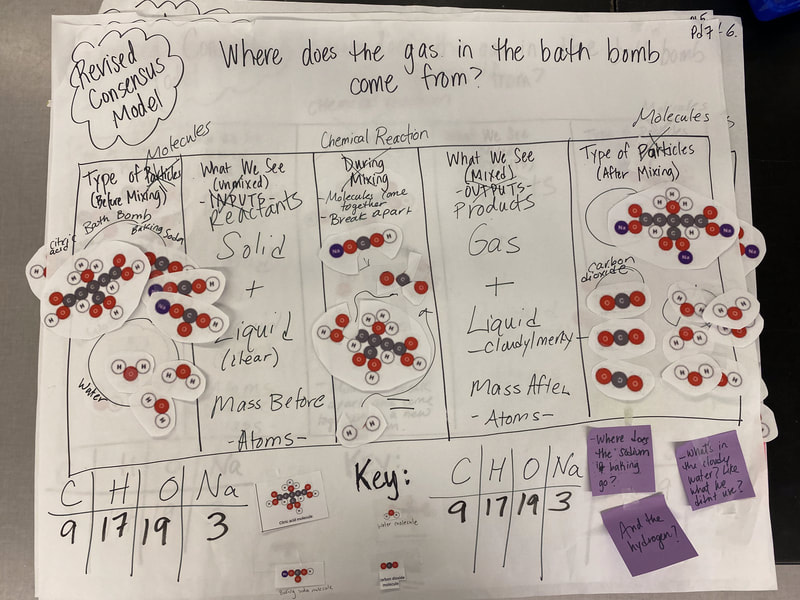
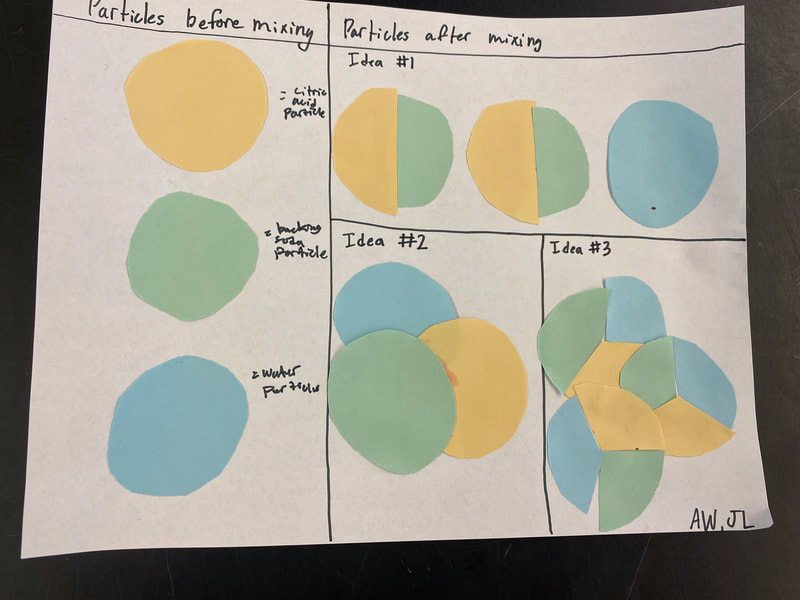
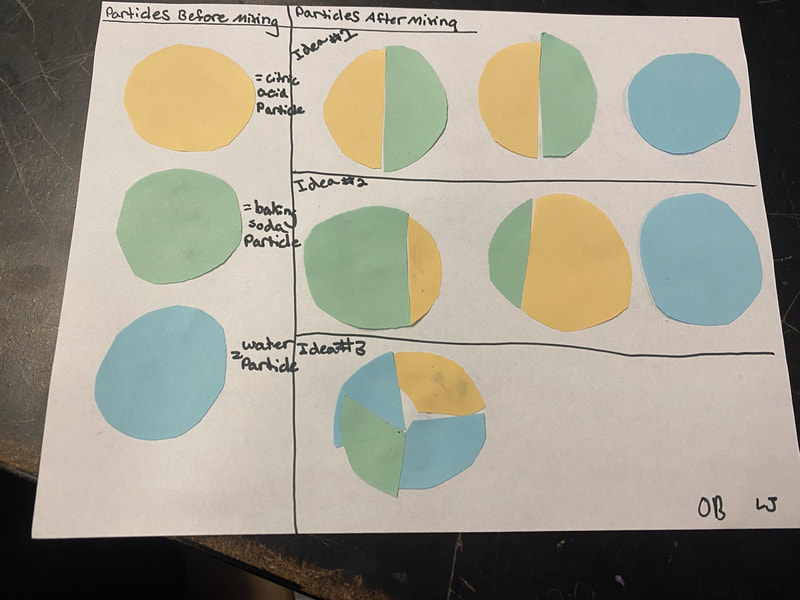
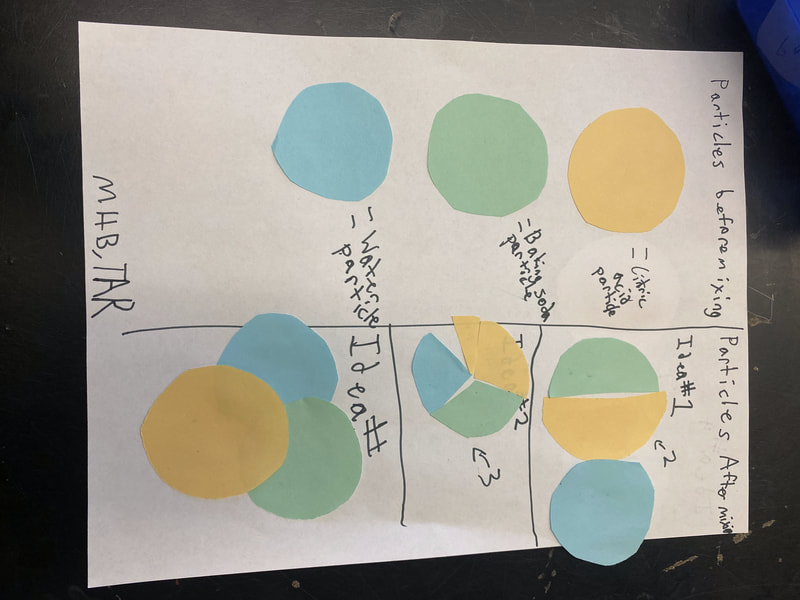
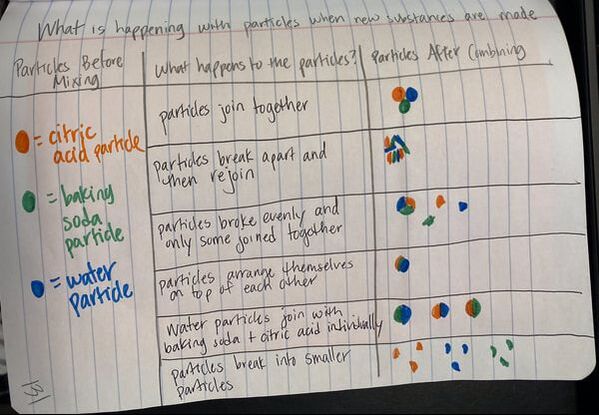
Now that we had evidence to suggest particles really were, we moved forward with representing our ideas no longer with particles, but instead with molecules. We had an awesome time building out various models of water as a start, and then moved towards understanding that different molecules are made of the same atoms but in different amounts, shapes and arrangements. From here, we started to think about how our chemical reaction starts with baking soda, water, and citric acid, and ends up with carbon dioxide! Check out some students' thinking as they tried to model this! And from here, we worked to really push for what a chemical reaction means, and how our reactant side of our chemical reaction must have the same atoms as the product side of our reaction! We've not only identified the mystery bath bomb gas, but we've figured out other products that included water and citric acid! Way to go 6th graders!
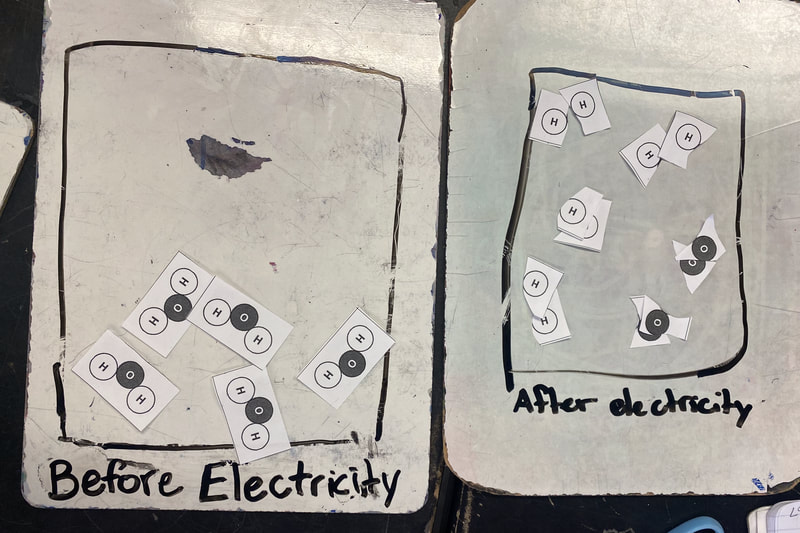
After testing the flammability of the two gases we produced from running electricity through water, we agreed that we've got two new substances! They behaved differently than the gas we created from heating water.
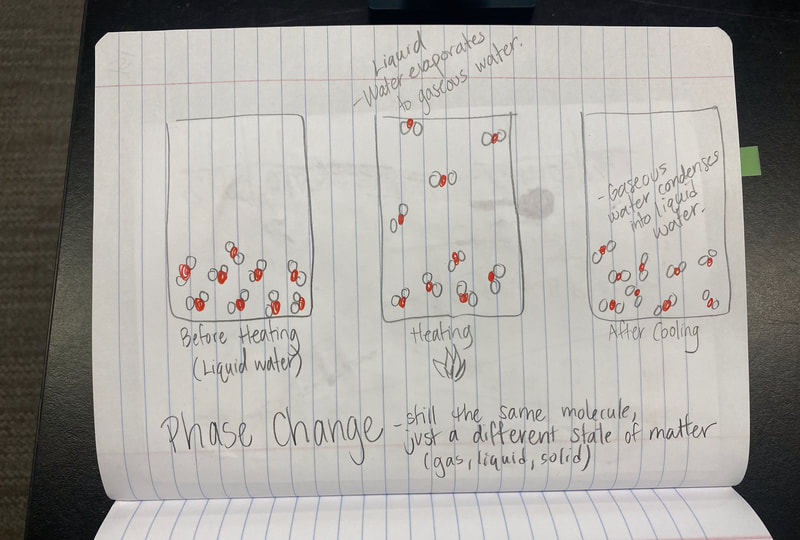
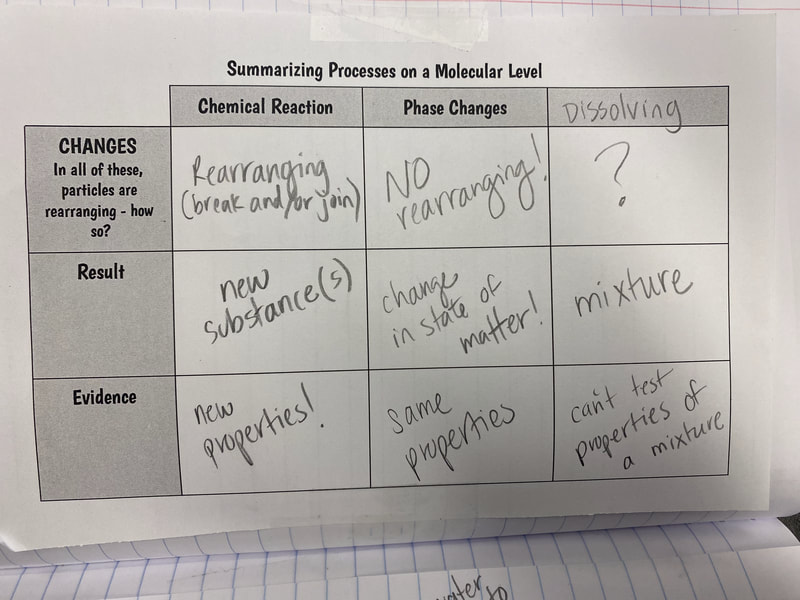
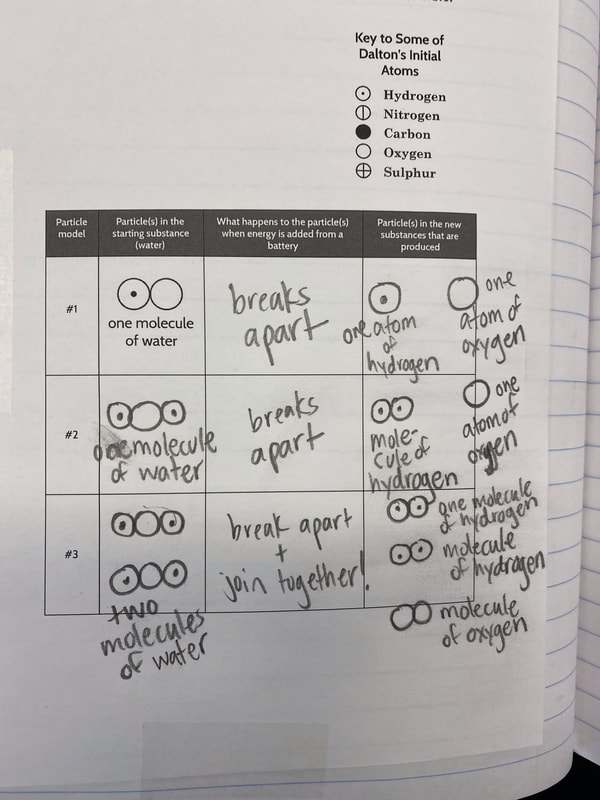
All this figuring out made us realize this. There are times we end up with a new substance, and there are time we don't. When we take a substance or substances and make something new out of them, this is what scientists call a chemical reaction. We realized that we can make new substances by combining things together (like with the bath bomb and water) or by breaking things apart, like we did with water and electricity. This was hard to wrap our heads around since we're so used to thinking about chemical reactions as just mixing things together, like liquids. But chemical reactions are so much more than that! We also turned to the work of other scientists yet again, as we were roadblocked on "particles." Our water investigations showed us that a water particle has to actually have "parts" to the particle, otherwise, when it's broken (like when we run electricity through it) we wouldn't have two new substances! Check out our work through reading and modeling. Next steps: dig deeper into our new understanding of atoms and molecules! So now that we figured out that heating water doesn't produce a new substance, we had to see if running electricity through water produced a new substance. Through our setup, we realized that bubbles were obviously being produced and they looked way different than the bubbles created from our bath bombs and our heating water investigations.
These gases also did very different things than either the bath bomb gas(es) or the heating water gas, which both extinguished a flame! These gases made a mini-explosion and allowed a burning ember to re-ignite! Seems like we have made new substances, both of which are different from each other and the bath bomb and heating water gases!
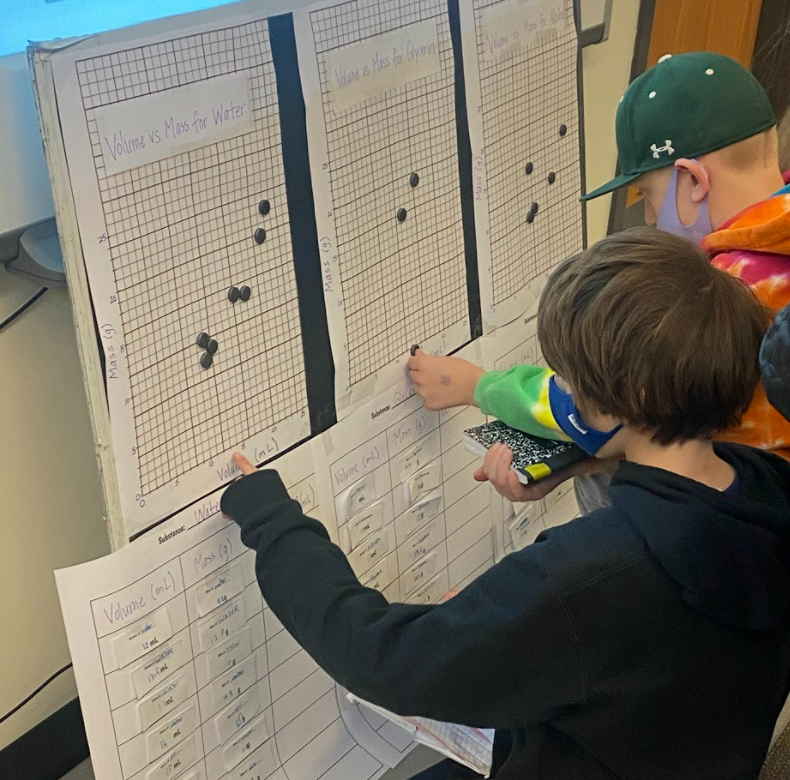
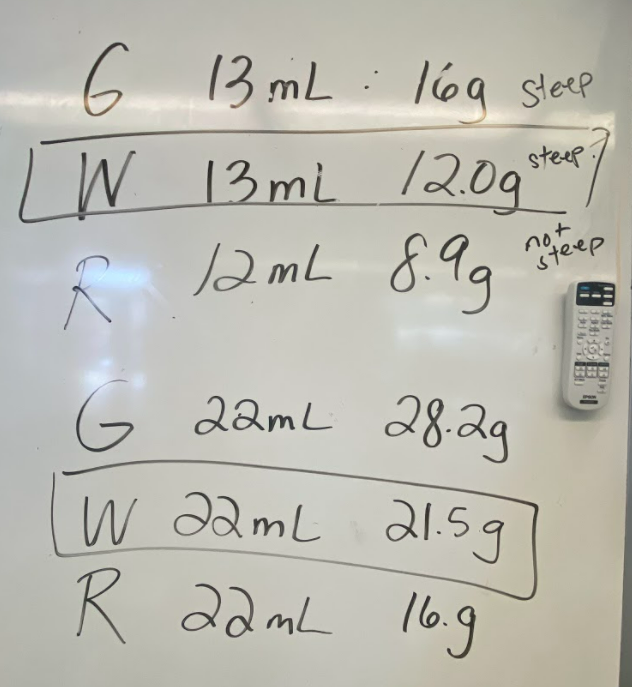
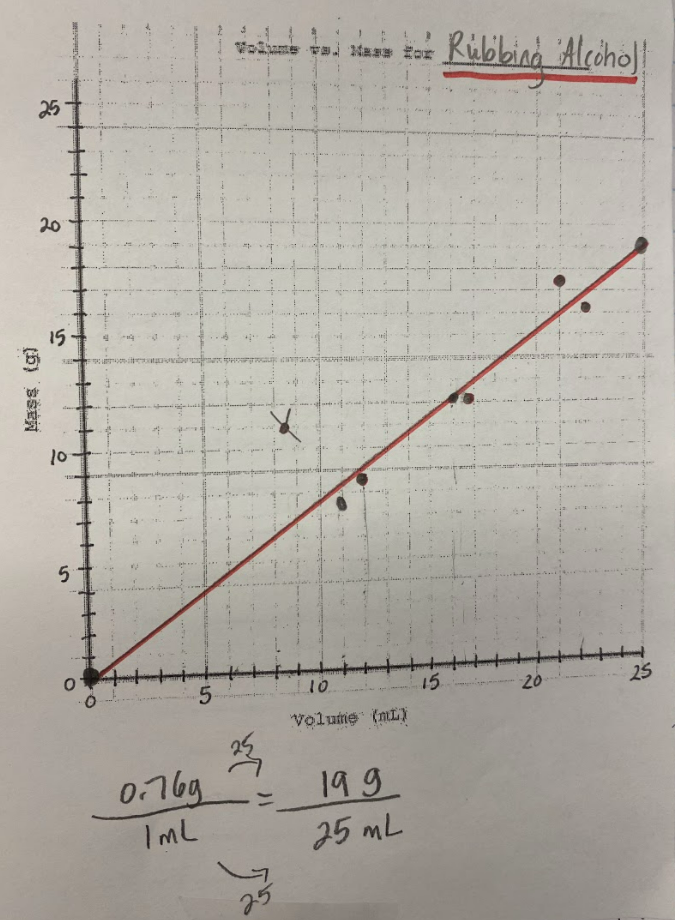
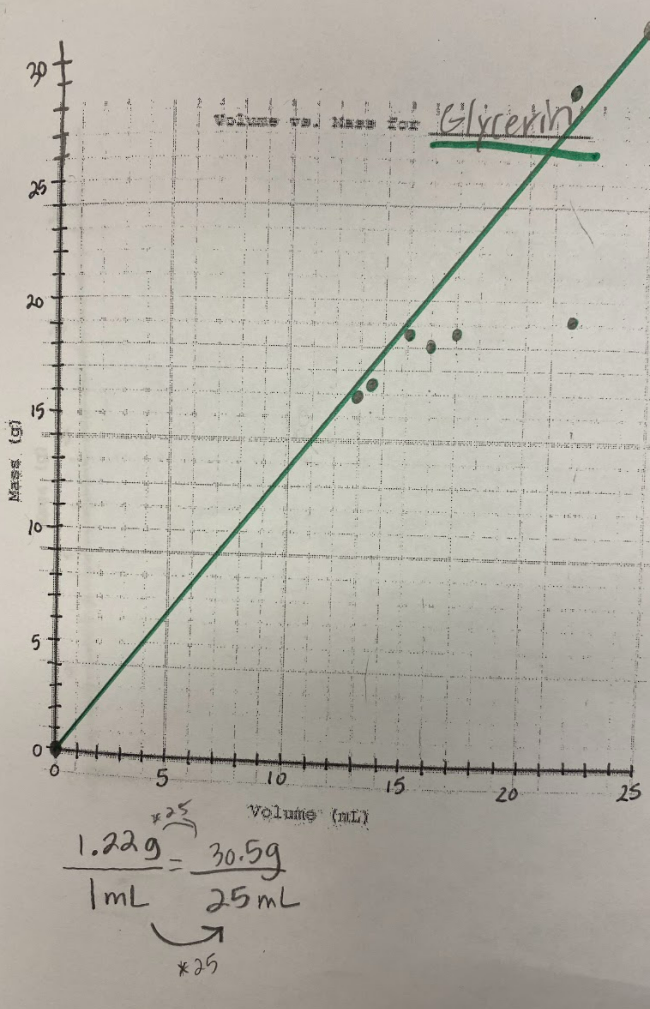
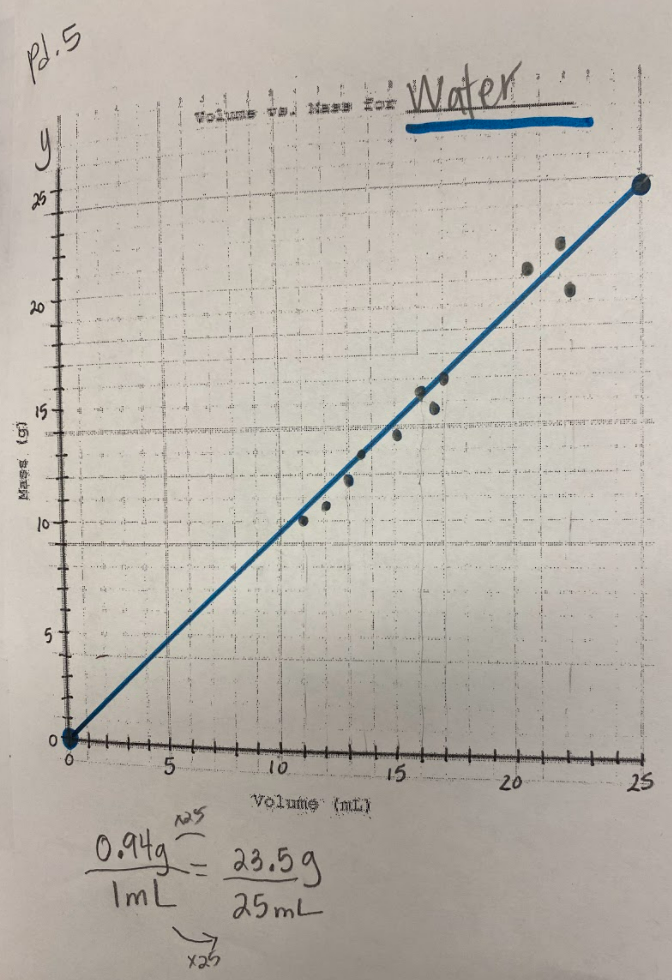
So what does this all mean? If we have new substances from the bath bomb interaction with water, and from running electricity through water, how do we actually get new particles from old particles? Like how do you actually create a new substance from an old substance if we agreed that you can either break apart or join particles together to make new substances??!?! We recognized through Dalton's work that we could get a gas by heating water. We didn't think that there would be a new substance, but we also saw that we needed to gather evidence to prove this true! We saw that heating water certainly produced bubbles, and the gas that was escaping a connected tube also was turning back into a clear substance. We wanted to see what would happen in the flammability test, and this gas extinguished a flame. We realized we couldn't test the density of the clear liquid when it was still hot, so we waited for it to cool down to room temperature. While we waited for the liquid to cool down, we realized we had limited understanding of what density actually was. Looking at the unit for density, we realized it was a comparison of g/mL, which meant it was a unit rate comparing the mass of a substance to its volume. We also began to see that water wasn't the only clear liquid around (duh!) and that the density of a substance is a great way to identify a substance that looks exactly like another one! Our efforts to see patterns in the mass vs. volume relationship began to emerge, and we saw that there were diagonal lines getting taller appearing, but at different rates. Rubbing alcohol's density was the lowest, with the least steep line. Glycerin's line was the steepest, with water's line falling in the middle! We also agreed that density was a unit rate, figuring out how much a substance's mass was PER milliliter of volume! By this time our clear liquid had cooled, and by weighing it and calculating it's volume, we determined the clear liquid's density to be 0.96g/mL. Since we calculated the density of water (when we knew it was water) to be close to this value, we agreed that heating water doesn't produce a new substance, and it's still made of water particles!
So to summarize, we don't end up with a new substance when we heat it! Our bath bomb gas is a new substance, so these processes are NOT the same! Onto running electricity through water! After agreeing that new particles could come from old particles either breaking apart or joining together, we revisited our investigation ideas to realize that we reached a roadblock. We didn't have any more investigation ideas that could help us figure out what the bath bomb gas(es) were or how they appeared. We were truly stuck!
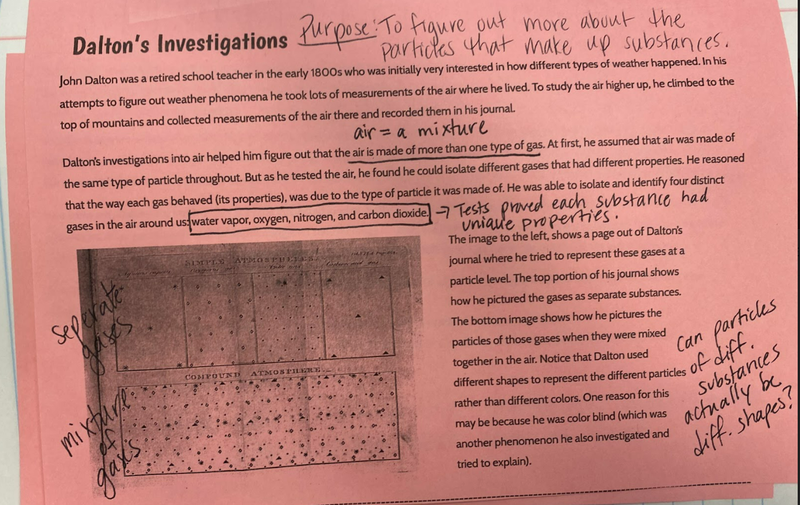
So we turned to the work of another scientist, John Dalton, who did some work with particles and gases that appeared. After reading an article about him, we updated our investigation ideas to include heating water and running electricity through water to see if we could end up with new substances with either of these processes! With all the talk about how we could get new particles from old particles, we had to use some creative thinking! Between splitting particles, to combining particles, to a combination of both breaking and putting together again, students are trying to truly think of ways we can get new particles from old ones. Way to go 6th grade!
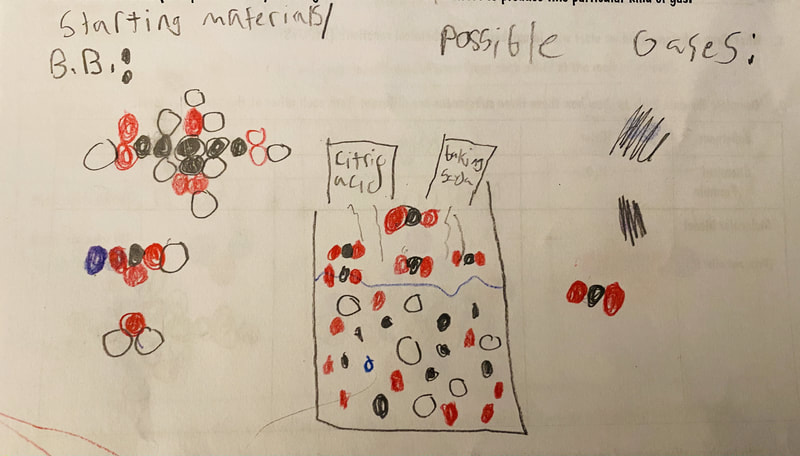
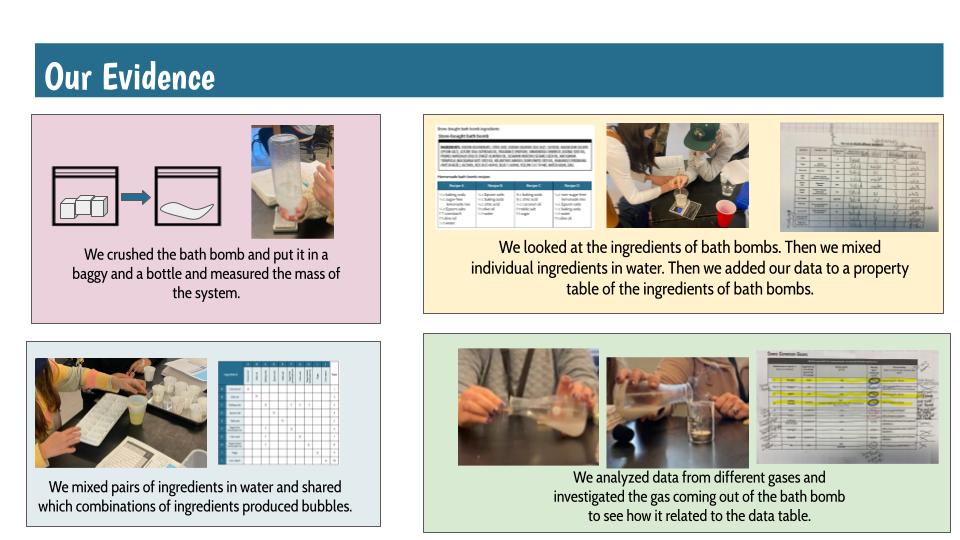
After a round of partner-share, we agreed we've gathered a lot of evidence to change our consensus models. We could eliminate that air was inside the bath bombs, and that we knew the bath bombs started as a mixture. The two key ingredients to the bath bomb reaction were citric acid and baking soda, and through various tests on the gas(es) produced, we could narrow it down to three because of their properties! Check out our new consensus models! All this discussion got us really confused, honestly. We agreed on a lot, but we also didn't understand how you could make a totally new particle if it wasn't there before. We left our discussion thinking about how this could happen alongside any lingering questions. Check out this snapshot of 6th graders' thinking! Next steps: Investigate how new particles can possible come from old particles!
|
Mrs. BrinzaSo how exactly doesn't something new get created when it wasn't there before? Archives
March 2022
Categories |




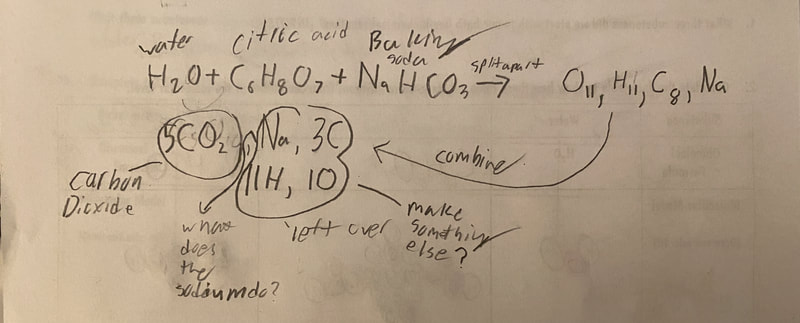
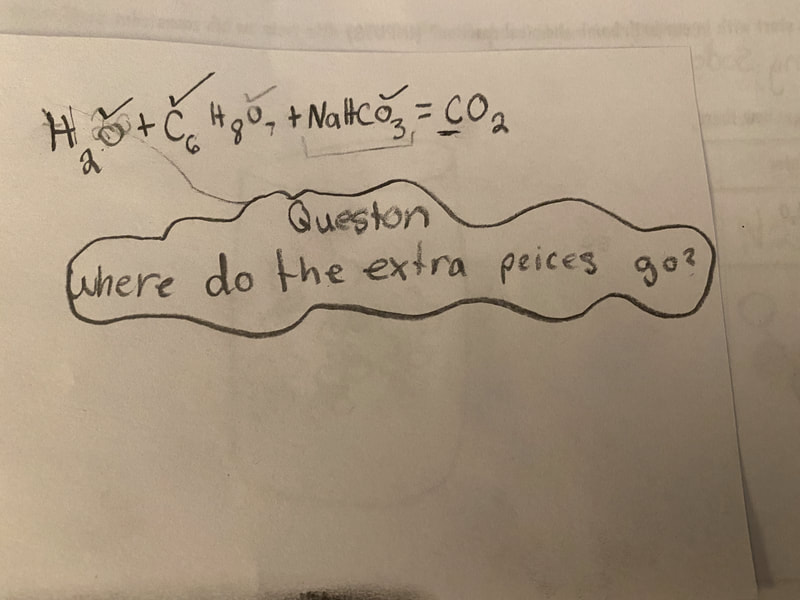














 RSS Feed
RSS Feed