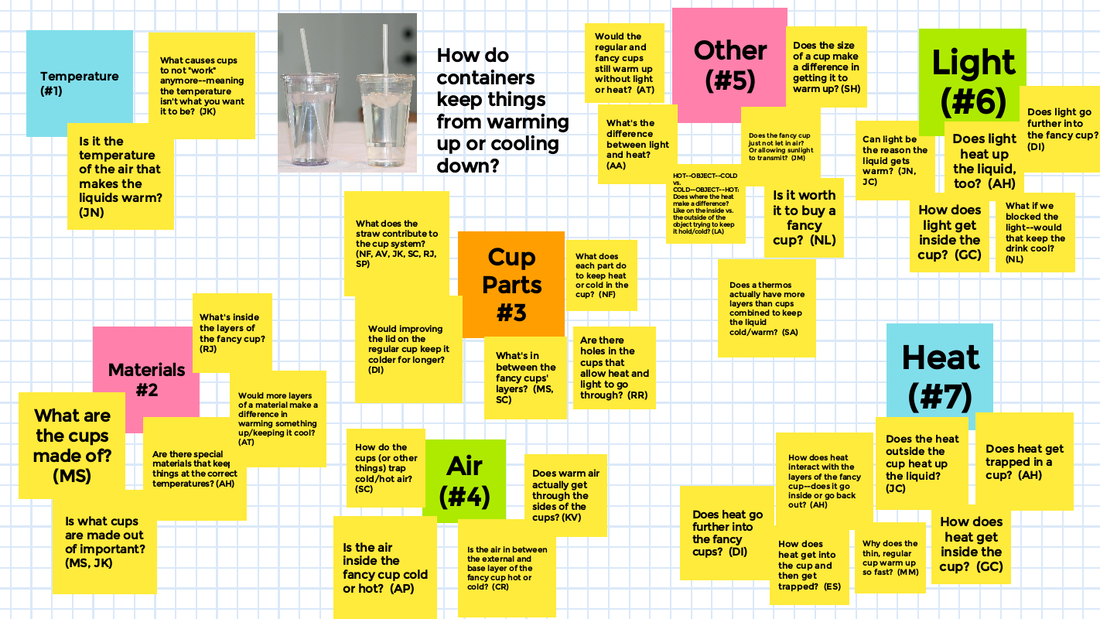
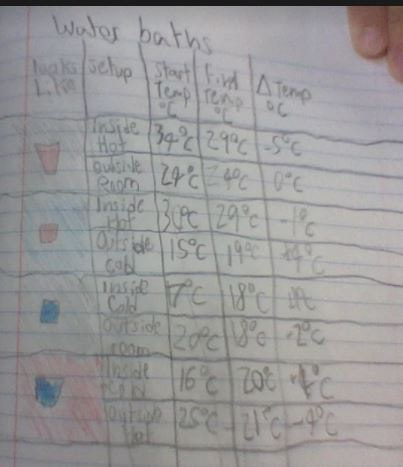
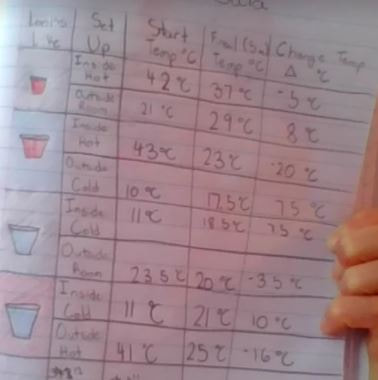
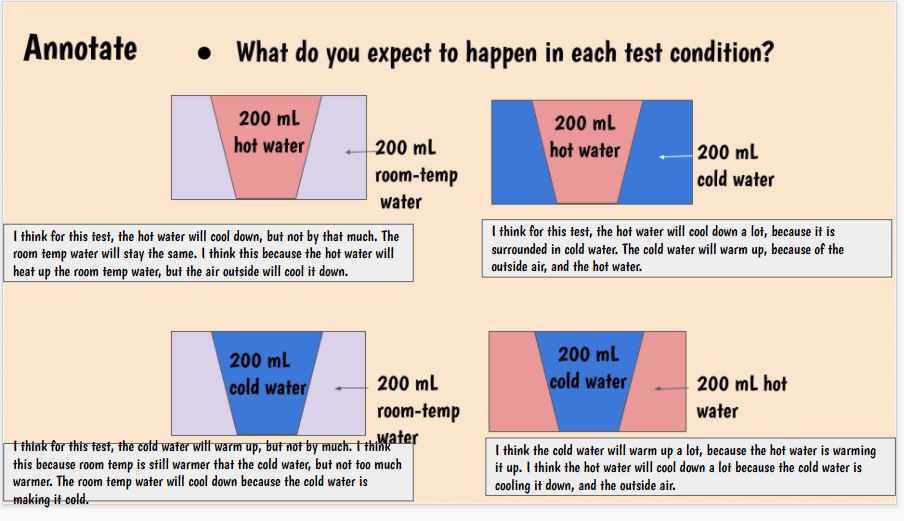
With all this figuring out happening, we turned back to our Driving Question Board to answer all the questions we had from the launch of our unit! Look at how far we've come!
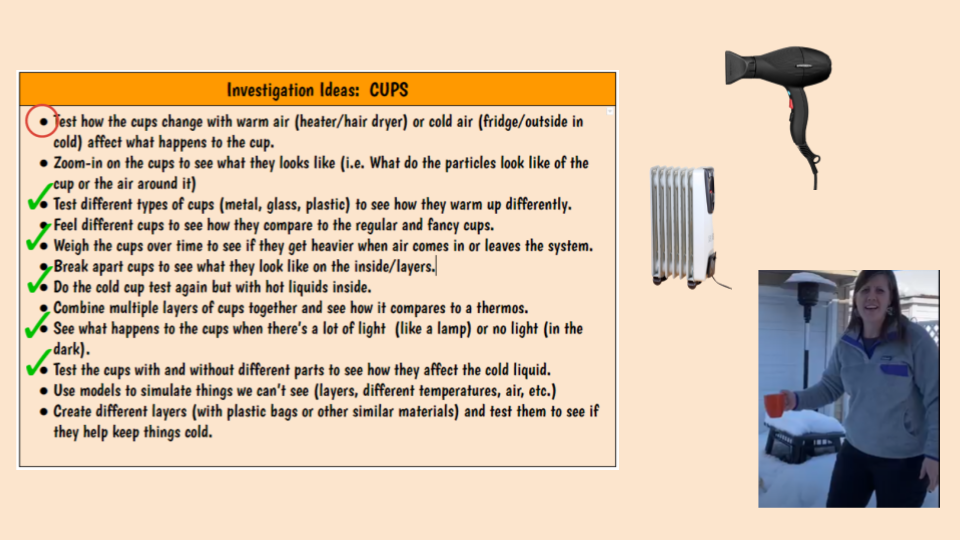
Mrs. Brinza also challenged the sixth graders to redesigning a cup with any features they felt would help slow energy transfer. Since we were in a combination of hyrbid and remote, we did the challenge on a day where everyone was remote! Students had to put ice in their redesigned cups at the start of class, and if anyone was successful with ice in their redesigned cups by 3pm when school got out, Mrs. Brinza would pie herself! Based on the pic below, do you think some of the students were successful? I think so! Way to go 6th graders!











 RSS Feed
RSS Feed