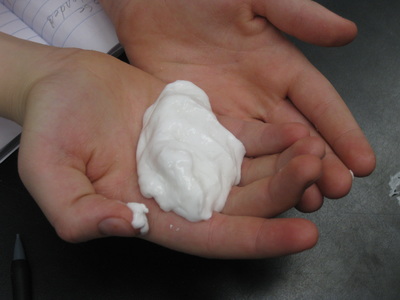
| | So students are understanding that mass can't be created nor destroyed, but why might we even combine two or more substances together in the first place? "To see what happens!" "To make something new." "To create something you need." Yup! You're right! We started discussing why substances might be combined, such as in baking, in medicine, or for technology purposes. So our last lesson in our chemical tests unit involved making mixtures for an intended purpose: to make a sole for an athletic shoe! Combining various chemicals gave us very different results...and students compared the results in a variety of tests. Check out their awesome work...and if you see them, ask them what shoe sole recipe they recommend! |
|
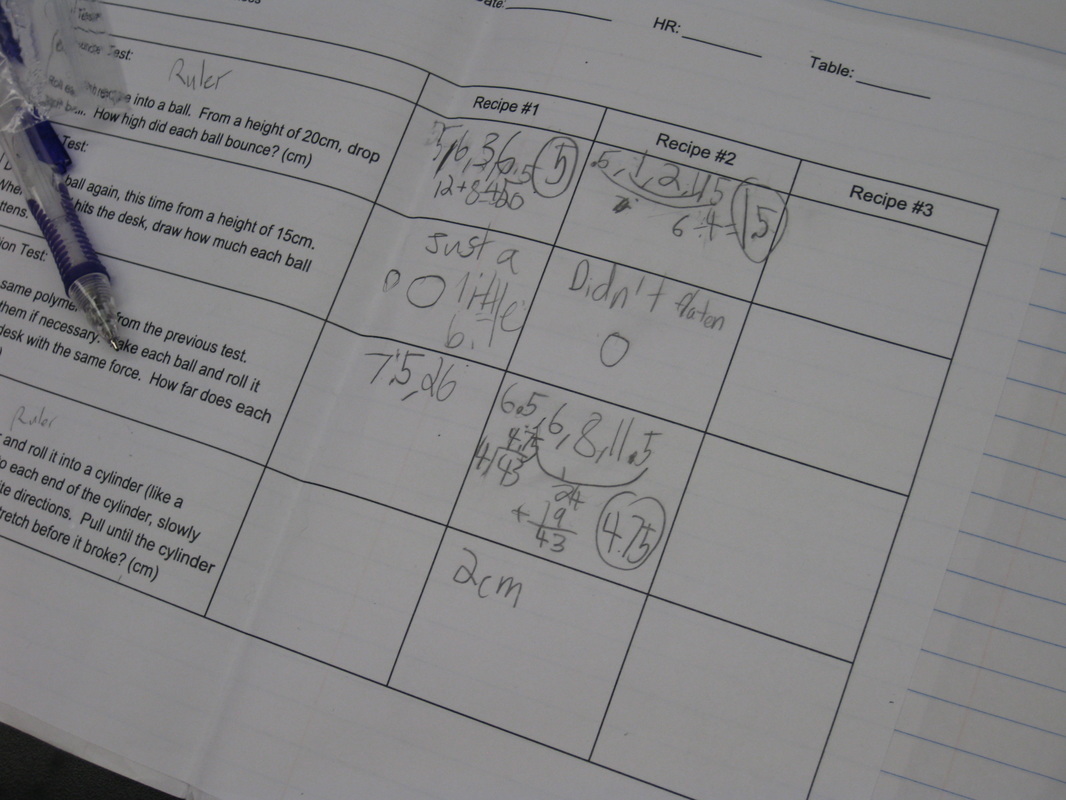
We reviewed the expected results of each of our four experiments, and students took off running showing their thinking. Check out the many different types of graphical displays students generated. How neat!
Students started to question if the gas that was inside the bubbles had mass, especially since over time, the bubbles popped and released the gas. Great wondering!
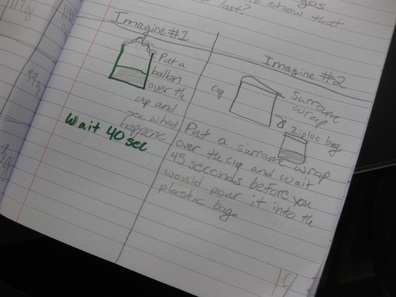
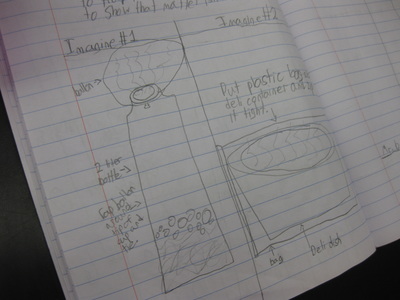
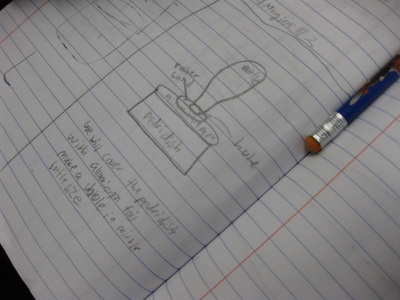
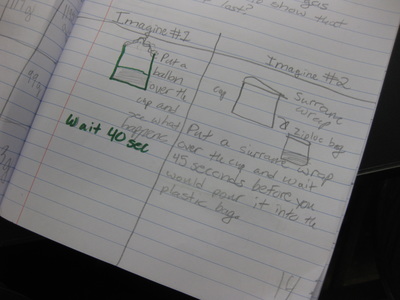
So we're engineering a possible solution to "trap" the gas to see if air not only has mass, but also to provide further evidence that the mass of a mixture should be the same as the mass of the substances that make it! Check out a small sampling of student's brainstorms to this problem. Next week, they'll be creating group plans and testing out their "gas-trapping technologies!" So fifth graders determined that even though they couldn't the sugar or the salt when they mixed it with water, it was still there. They proved that no one took out either chemical, and that letting one of them sit out overnight gave proof that it was still there. But is there another way to prove this phenomenon, that sugar and salt are still in the water, even though we can't see them?
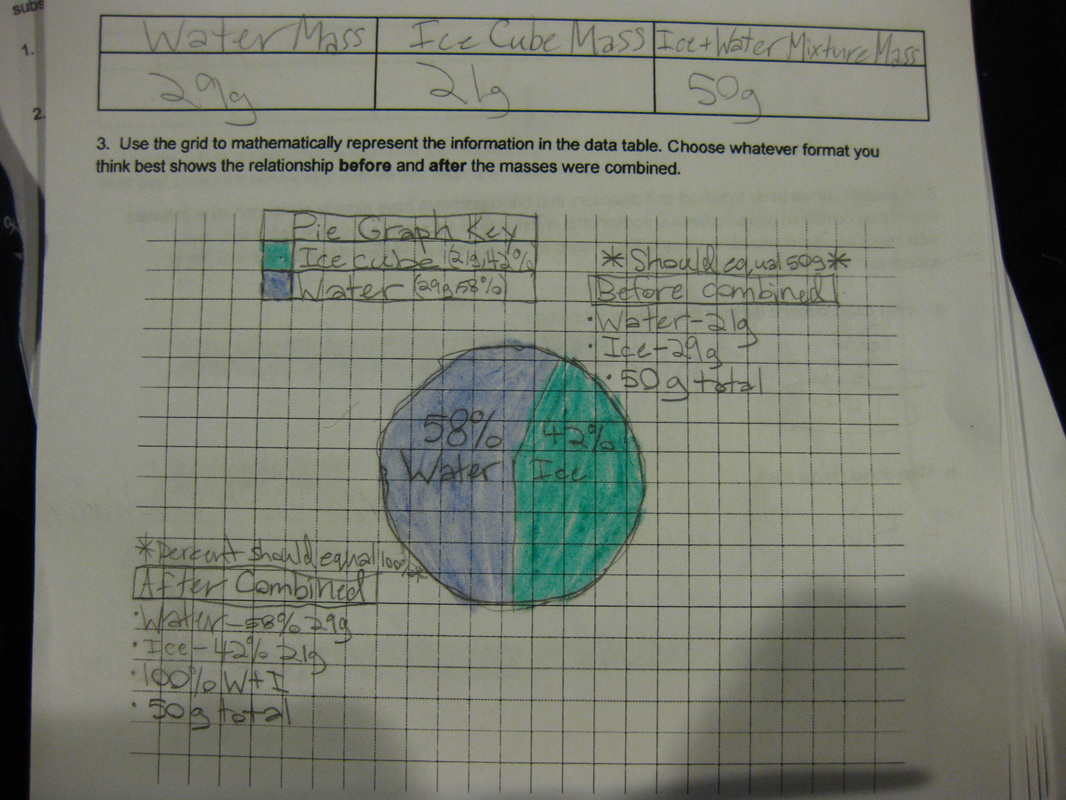
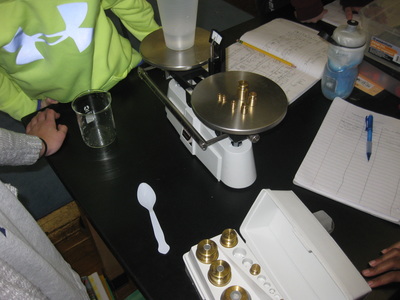
Using a two-pan balance (and a digital scale to see how accurate they were!), fifth graders designed an experiment to see if the mass of one of the chemicals (i.e. salt) added to the mass of another chemical (i.e. water) would equal the mass of both together. If the mass of the two combined equaled the mass of each of the two seperately, this would give more evidence that even if we physically couldn't see them, that they must be there. Great work fifth graders! So if sugar dissolves in water, what will happen if salt is placed in water? What kind of evidence can we gather to support the idea that it will probably dissolve, too? Students had limited materials and spent Thursday of last week designing their own experiments. We checked back on Tuesday to to collect data and think the long weekend might help to gather more data! Each group discovered that after a long weekend, the salt remained behind, and even though it looked different than the salt that was initially put in the water, it was still there and didn't "disappear!" So even though we couldn't physically see the salt in the water when we mixed it, we knew it was there after we let the water evaporate!
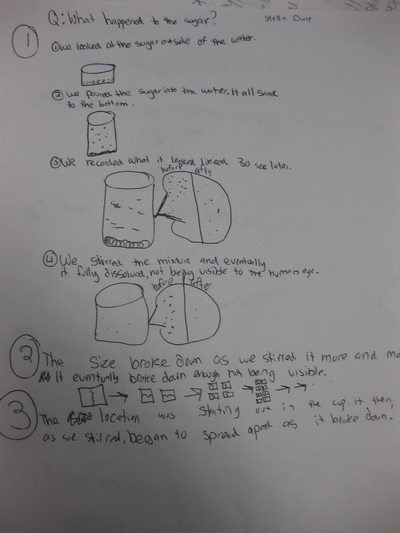
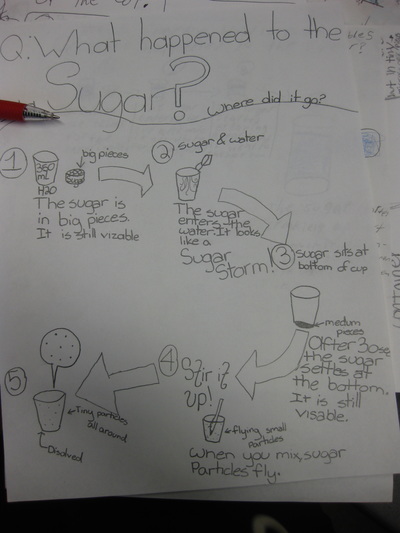
Fifth graders are jumping back into studying chemistry by exploring what happens to different substances when we combine them together. They worked to answer the question "What Happened to the Sugar?" when they placed a spoonful of sugar in water. This was one of the mystery substances they uncovered in the fall.
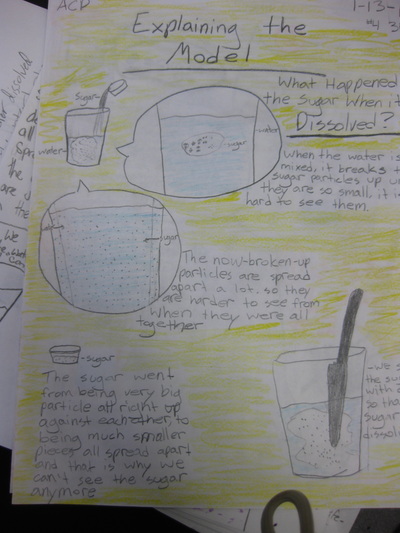
Using evidence, students developed a model to explain this phenomenon. Did the sugar disappear? What does it actually mean to dissolve something? How do we know that the sugar is still in the water even if we can't see it? Students developed a model to show how the sugar dissolved! We discussed how if the sugar was still in the water,
This week, students used the evidence they gathered from all their tests to determine the identities of the mystery chemicals. Students were provided with a chemicals information sheet, and they used the Claim-Evidence-Reasoning model to determine which chemical was sugar, baking soda, alum, talc, and cornstarch. Check out their awesome work!
|
Mrs. BrinzaWe always practice safe science in our room. We wear goggles to protect our eyes, never touch anything that is unsafe, and keep the aisles clear so we can freely and safely move around the room. Archives
February 2016
Categories |































 RSS Feed
RSS Feed