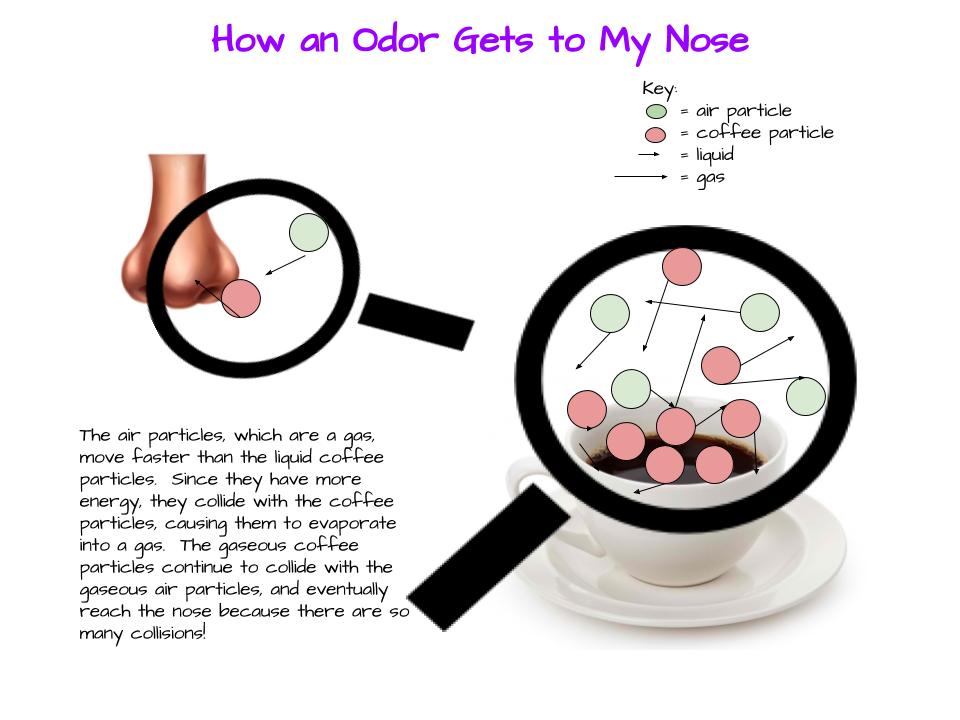
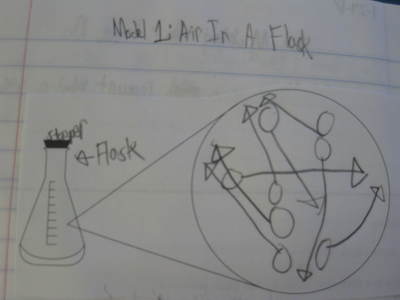
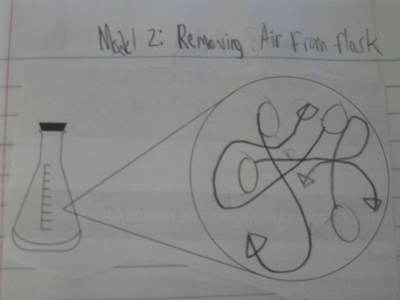
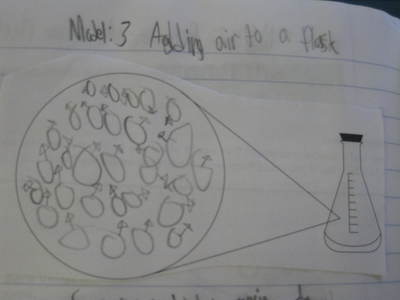
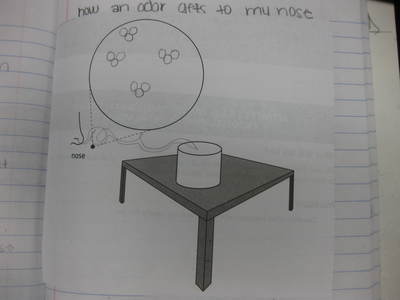
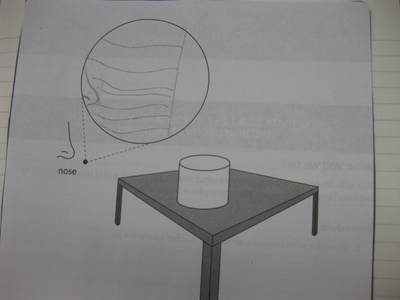
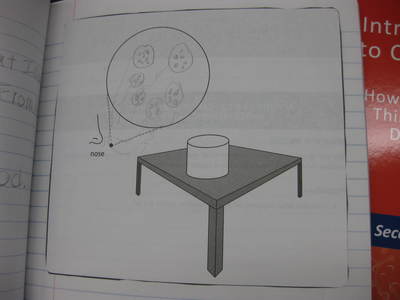
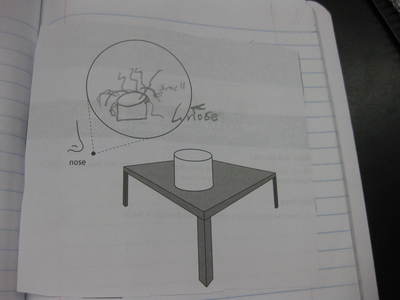
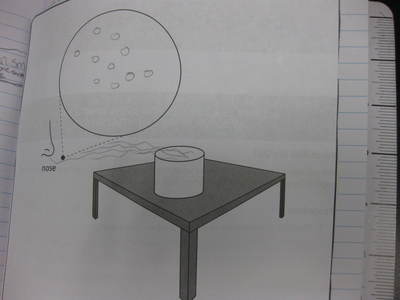
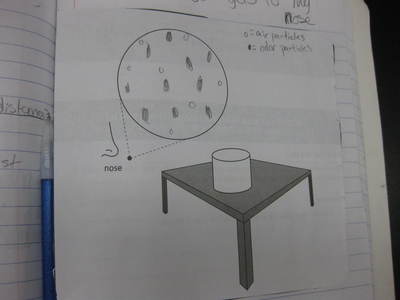
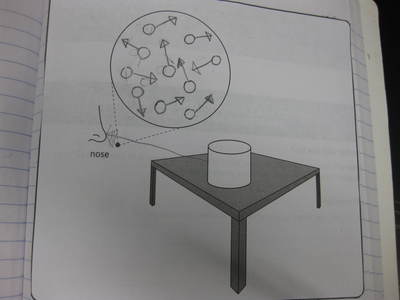
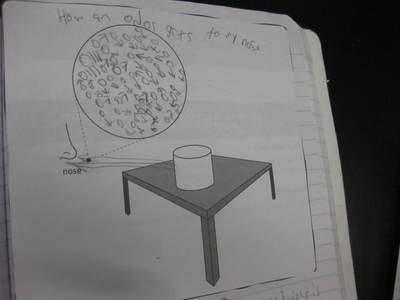
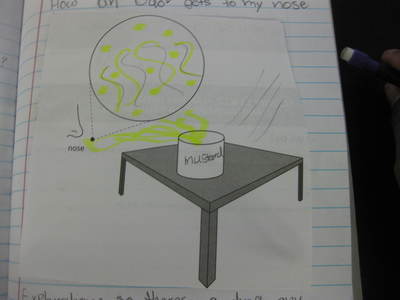
After some thoughtful discussion, we've established consensus for how an odor gets to our noses. Air plays a pivotal role, especially as we haven't applied any heat to our liquid that we smell. Collision also plays a role! Check out our original thinking below...does each group's model show air particles, odor particles, phase change, and particle movement? We had a great time evaluating each other's work because we came to consensus!
|
Sixth graders used these two simulations to help further develop their thinking behind how an odor can get to their nose!
Click to set custom HTML
In our last demonstration, we learned that gas can be added and removed from a container. We saw this as the stopper on top of a flask was sucked in and pushed off! Because gas is made of matter and has mass, the mass of the flask increased as gas was added and decreased as gas was released.
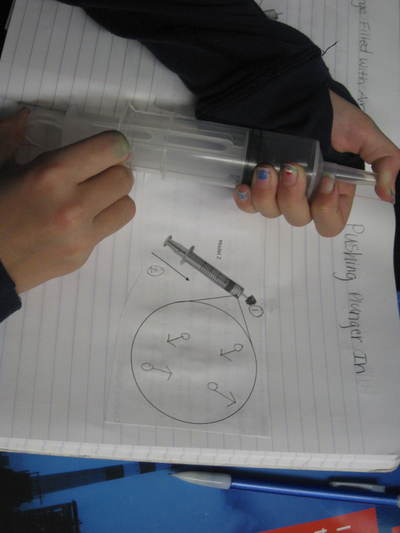
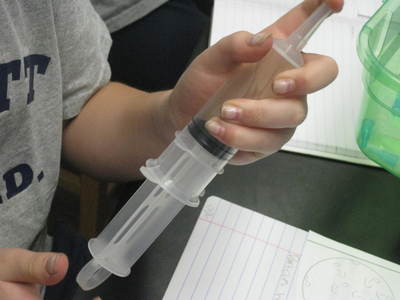
But today's lab involved students keeping the mass constant and changing the volume. Increasing the volume of the syringe allowed students to see that air can expand and spread out. The mass didn't change because no air was added to the syringe to therefore change its mass. When the volume of the syringe decreased, the air inside was compressed, and moved into a smaller space. The mass also remained constant because no more air was added or removed from the syringe. We'll slowly discover how the ideas behind air's ability to expand and compress relates to why we can possibly smell an odor from a distance. Check back soon!  So if we smell something because it's in the air, what exactly can air do? Using a special flask and pump, students saw that air can both be added and taken away from a closed system. This allowed us to understand that there must be empty space around the air, and that that space changes when there is more or less air in a system. We're applying our knowledge of volume and mass to these situations too...it's all coming full circle! As sixth graders are trying to answer the driving question of how they smell something, it's important to see how phase change can play a role in all that! Using water as a second example (we first looked at menthol change phases), we're beginning to see how solids become liquids, and liquids become gases and then back again. We noticed how there was a change in volume when liquids became gases, providing evidence that some of the liquid MUST have evaporated into a gas.
But how do all these phase changes relate to how we can smell something? We noticed we smelled menthol MORE when it changed from a solid to a liquid, and EVEN MORE as it became a gas. Here were some great questions from today's demonstration and discussion: 1. Why don't we smell the water when it evaporated like we did the menthol? 2. Are some smells stronger than others? What makes them stronger? 3. Does everything have to become a gas in order for me to smell it? Hmmmm....great thinking! In a demonstration around menthol, students saw firsthand how menthol, which has a fairly strong odor as a solid, had an EVEN STRONGER odor as it was heated. Students walked through an initial discussion of how matter changes from one state of matter to the next, and are starting to see how this relates to our overall driving question of "How can I smell things from a distance?"
We heated the menthol, allowing it to change from a solid, to a liquid, and to a gas, and with the help of ice, cooled the menthol from a gas, back to a liquid, and then to a solid again. We'll be using the ideas from this demonstration to further develop the model to explain the "how we smell things" phenomena! Students have uncovered that air takes up space. Check out the photos above to see the evidence!
If air takes up space, then how does this relate to why sometimes I smell something and why other times I don't? What is air's role in why I smell something in the first place? What are those air particles really doing? As students develop models to explain the "how we smell" phenomena, it's important to take a deeper look at matter. Just what is it? How can it be described and measured? And how can our findings with the obvious (solids and liquids) relate to the less obvious (gases)? Students have uncovered that the odors they smell must be a gas, and that the odor particles must be different than air, but how exactly do they work together? Only time will tell before students uncover this!
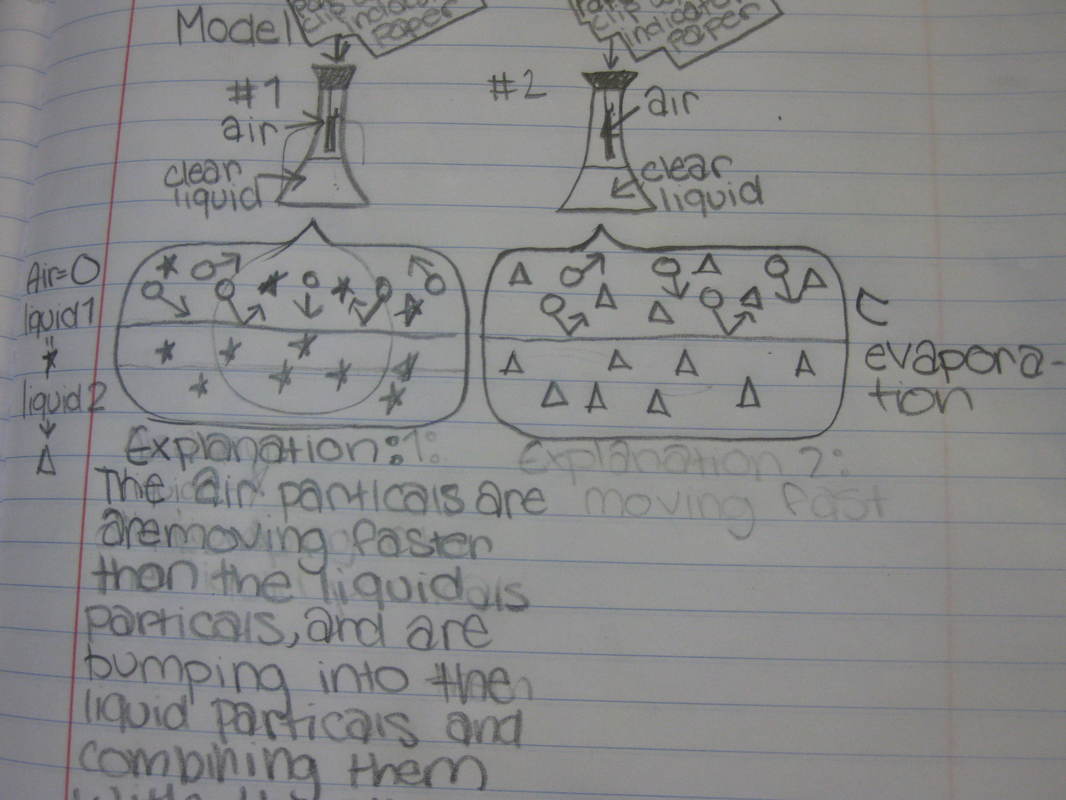
Students completed labs this week to solidify their understanding of mass and volume--great work 6th grade! So we all agree that an odor travels to our noses. But what exactly does that mean and look like? Here are some snapshots of our initial thinking! I wonder if our models will change as we gather more evidence!
|
My Favorite Smells1. Bread baking. Archives
March 2017
Categories |






















 RSS Feed
RSS Feed