An important aspect of understanding what happens as gases are heated and cooled also relates to the air around them and its temperature. Watching two videos on how carbon dioxide warms up and cools down, the gas was contained in a rubber balloon. It's obvious from our previous knowledge that the balloon expanded and shrank. But the air around each of the balloons was doing something, too, which allowed it to do so.
|
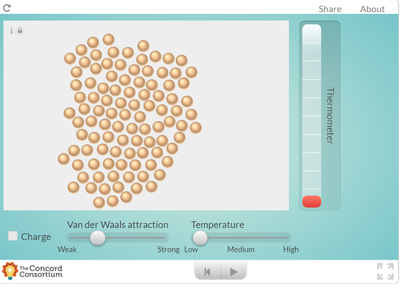
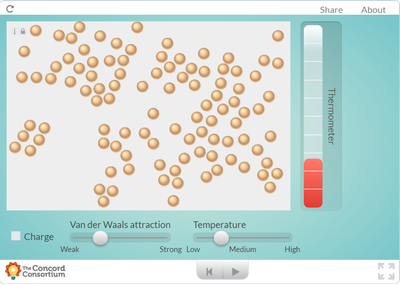
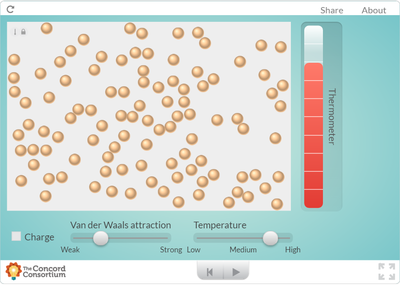
Our two dimensional models and ball-and-stick models do not show how the molecules in a substance are in constant motion. But a computer simulation will!
Using an online program, students saw what happens to molecules as they are heated. How does heating a substance affect its motion? How does it affect the volume of a substance? Take a look for yourself... But it seems like when there is more energy in a substance, the molecules move faster and take up more space. Is this why I smell a hot pizza quicker across a room than a cold pizza? Hmmmmm....
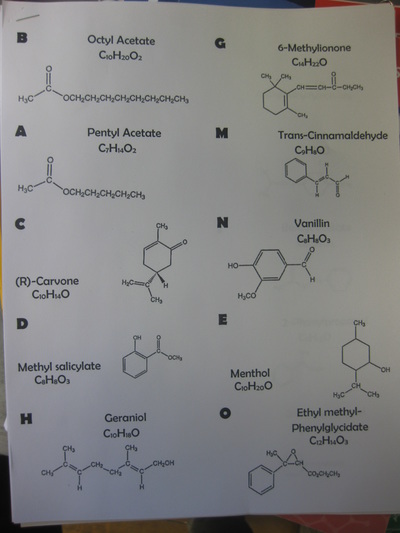
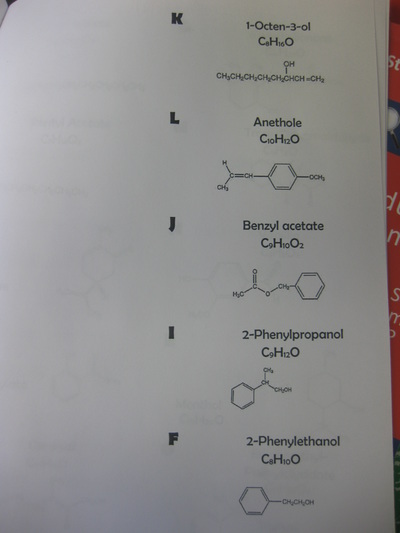
 Our findings: It took significantly less time for the warm ammonia to change the indicator paper than the cold ammonia. How will this help us explain how we can possibly change a substance in order to smell it? Only time will tell!  So as students uncovered that air is comprised of different molecules, we're uncovering how this idea can be connected to different odors. How are odors different from one another? By smelling various smells, students are also seeing the atoms that make up a molecule of each odor. We'll be looking for patterns to distinguish one smell from another, as well as see if the molecules that determine similar smells are somehow related, too. From our investigation, we discovered three big ideas!
1. Different substances can be made of both the same types of atoms or of different atoms. 2. Substances that are made of the same types of atoms differ in two ways: either the number of atoms or the ways in which they are arranged. Great thinking! 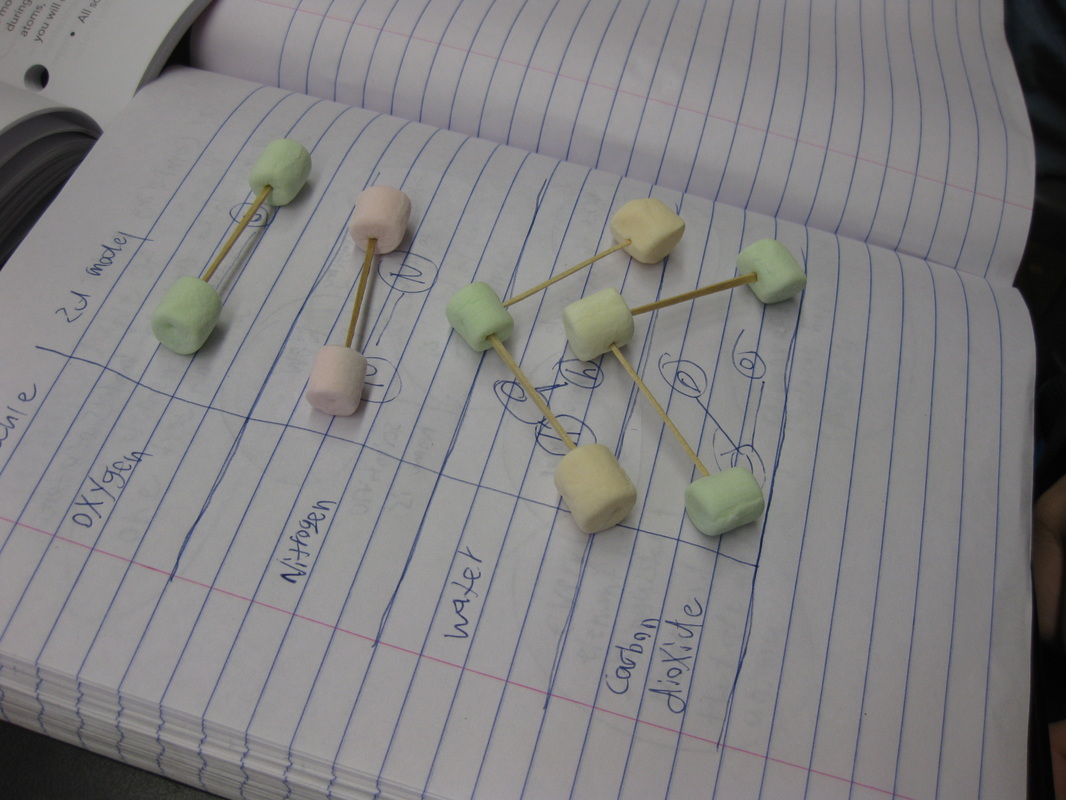 Comprised of many different gases, and each gas is actually a molecule, or a combination of at least two atoms. Since every gas has different properties, our idea behind particles being different makes perfect sense! We built gas molecules out of different colored marshmallows, recognizing their differences. While these models help us to see gases as different, they are limited. They don't know movement and are so incredibly small for what they really represent. I wonder where this will take us in our study!  Sixth graders recently looked at various metals, all of which are defined as elements. Elements are all made of the same atom throughout. Therefore, gold is made of gold atoms, and hydrogen is made of hydrogen atoms. We briefly looked at the Periodic Table to recognize patterns between the elements, and ultimately, tried to see similarities between all the elements near one another. It's getting us thinking for sure! As we dig deeper into understanding the basics of chemistry, students are recognizing that everything has different properties because it is made of different particles. These particles are really atoms, which make up all matter. Since different atoms are structured differently, this is what must make each element unique. All this learning is helping us relate back to our driving question of "How Can We Smell From a Distance?" and our smaller sub-question now of "How Is One Odor Different from Another?" It's all starting to come together now... So if different odors are made of different particles, and odors are gases, is it fair to say that other states of matter are made of different particles, too?
Using various metals (zinc, aluminum, iron, and copper), students are recognizing that these metals have unique properties that define them, too. Between their color, hardness, and malleability, they are all unique. What must this tell us about the particles that make up each of them? Hmmmm....I wonder! ;-)
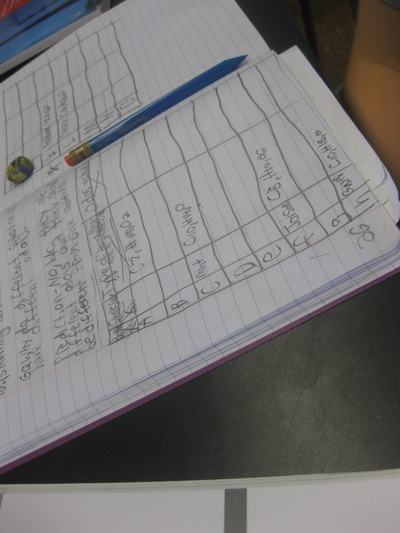
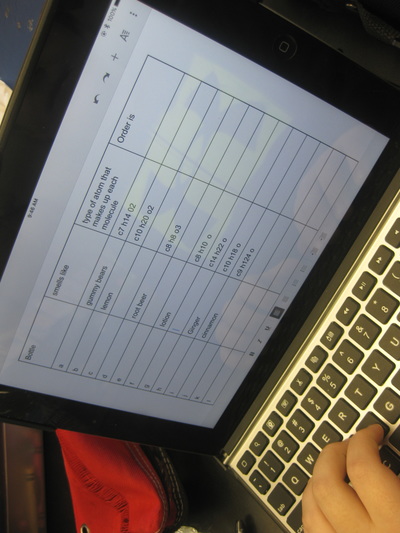
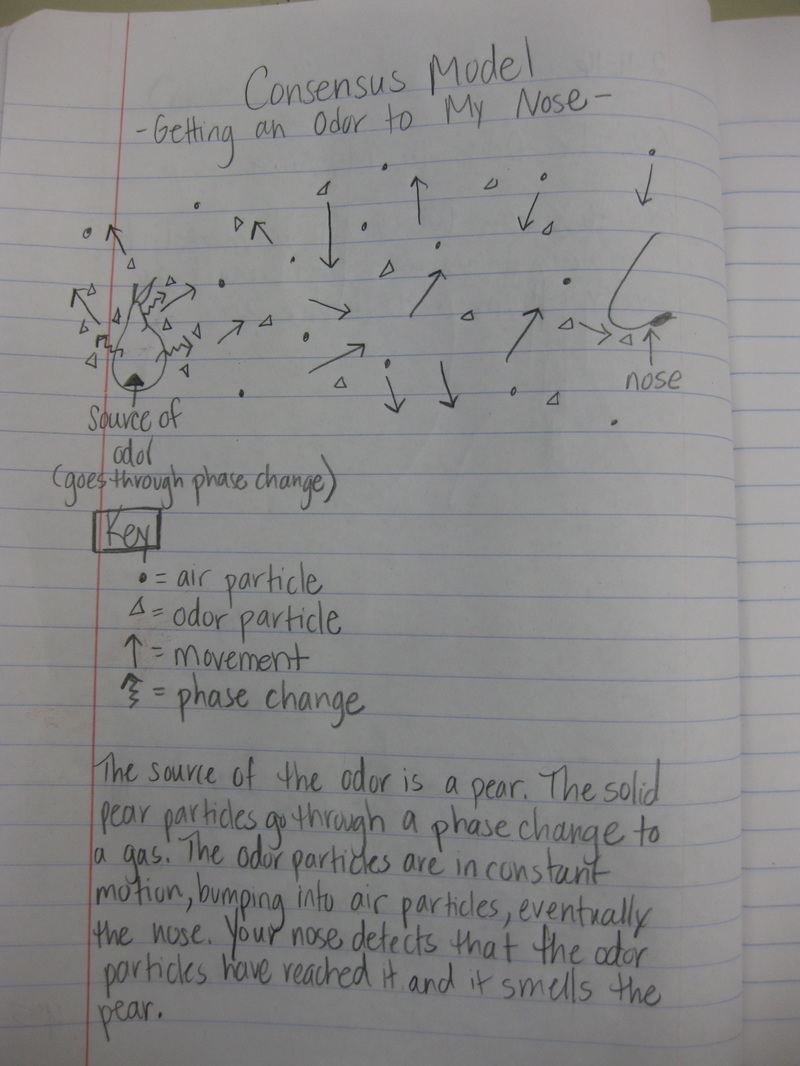
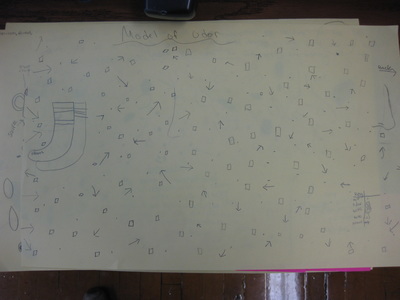
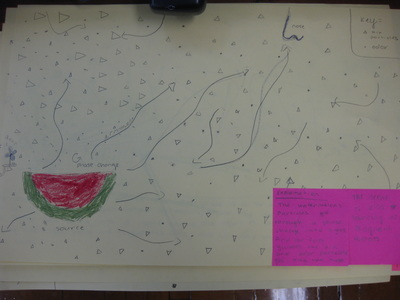
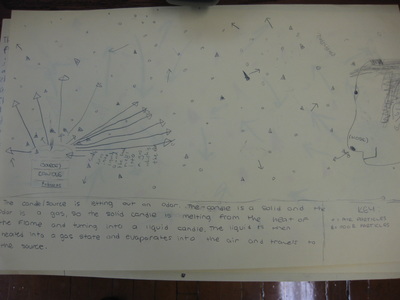
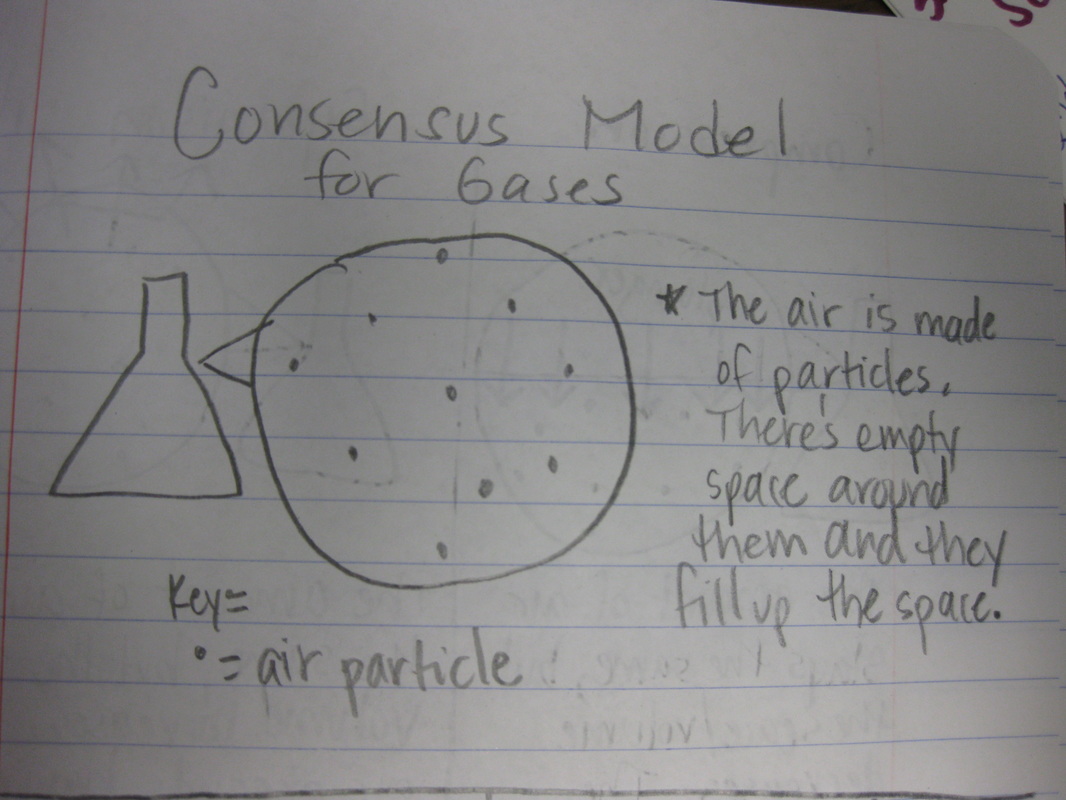
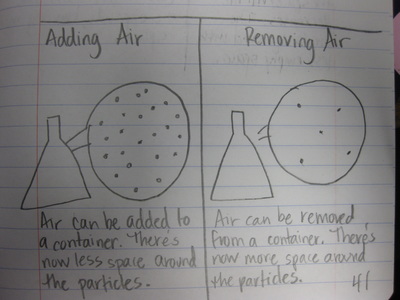
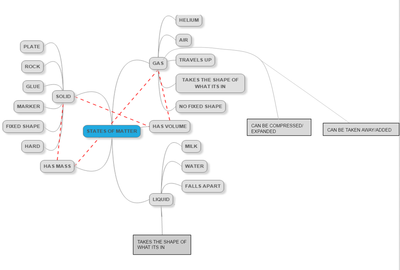
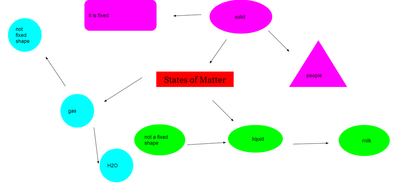
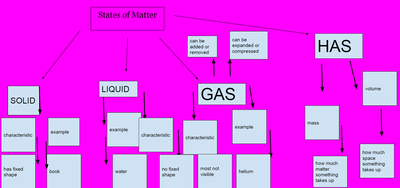
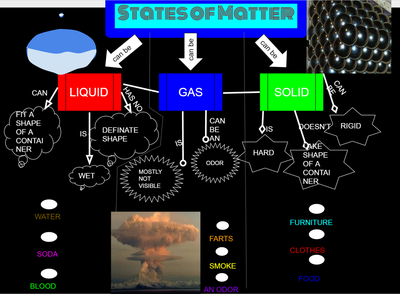
After quite a few students shared their thinking with the whole class, we worked through what our "consensus" should be. Great work (below is Mrs. Brinza's journal for what we agreed upon). As students complete their States of Matter Concept Maps for our technology integration class, they'll be adding these ideas to their concept maps. Here's a sneak peak into some of their work!
|
Mrs. BrinzaThree Favorite Smells: Archives
April 2016
Categories |













 RSS Feed
RSS Feed