To officially-officially wrap up our unit, sixth graders made personal add riddles for various terms used throughout our "How Can I Smell an Odor from a Distance?" To make you laugh...here were some of the simple statements they made...
"I like to skip class, so if you don't, then we won't be a match."
-Sublimation (because it skips a going from a solid to a liquid before it becomes a gas)
"I've got tons of energy, so if you aren't willing to move with me, then we won't be good together."
-Gas (because the particles are moving the fastest out of the three states of matter)
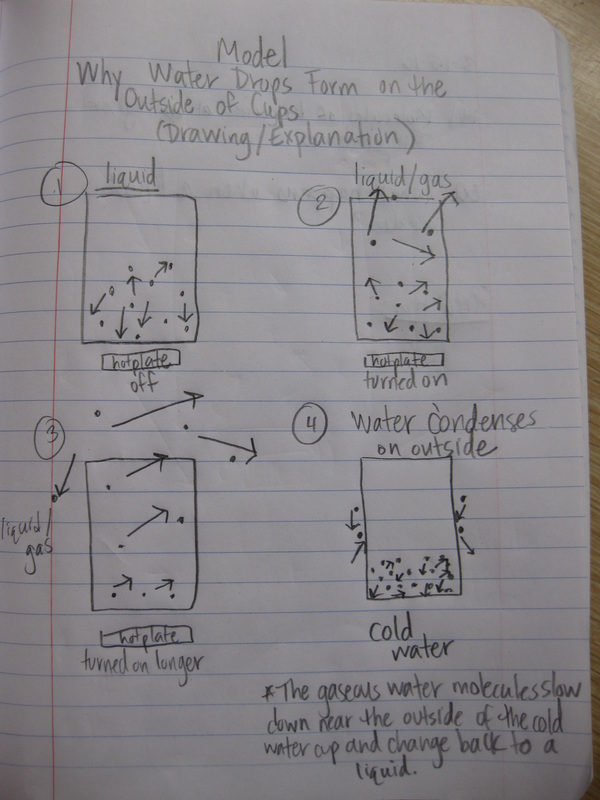
"I am looking to slow down in life, so if you're not into doing this, don't even bother contacting me."
-Condensation (because the particles slow down during this phase change)
"I like to skip class, so if you don't, then we won't be a match."
-Sublimation (because it skips a going from a solid to a liquid before it becomes a gas)
"I've got tons of energy, so if you aren't willing to move with me, then we won't be good together."
-Gas (because the particles are moving the fastest out of the three states of matter)
"I am looking to slow down in life, so if you're not into doing this, don't even bother contacting me."
-Condensation (because the particles slow down during this phase change)


















 RSS Feed
RSS Feed