Sixth grade has come a long way to explaining their driving question of how they can smell things from a distance. By using the scientific practice of modeling, they've uncovered a LOT of science content that explains this phenomenon. From particle theory, to phase changes, to molecular structure, they're on a role with understanding basic chemistry principles they'll revisit in later years. Great work 6th grade!
|
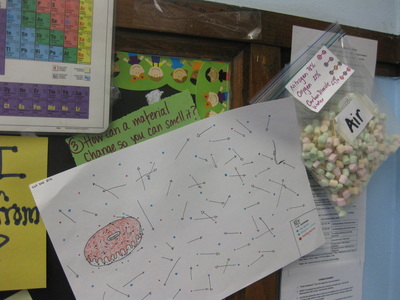
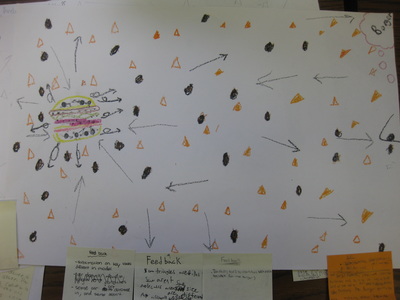
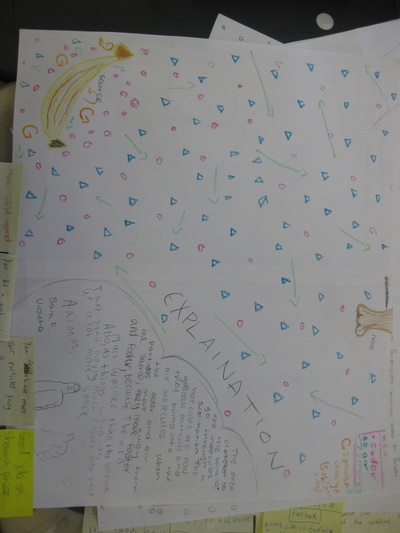
So we're nearing the end of our unit on how we can smell an odor from a distance, and we're finally able to put it altogether! Check out the models we've built as a class, and compare each one. In a culminating activity, we built consensus in discussion to explain this phenomenon. Great work sixth graders! I bet you'll never think the same way about how you smell something ever again!
We smell menthol and it doesn't seem to melt. We smell play-dough and deodorant, too, and they don't seem to melt and then evaporate. So is it possible that some solids can completely skip the melting/evaporation phases and go straight from a solid to a gas?
The answer is yes! And the process is sublimation. When solids change from a solid to a gas, they sublimate. Much of what we smell is because of this process. We'll be using this process to help explain why we smell an odor from a distance...
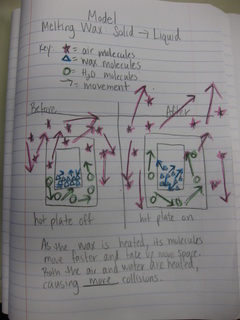
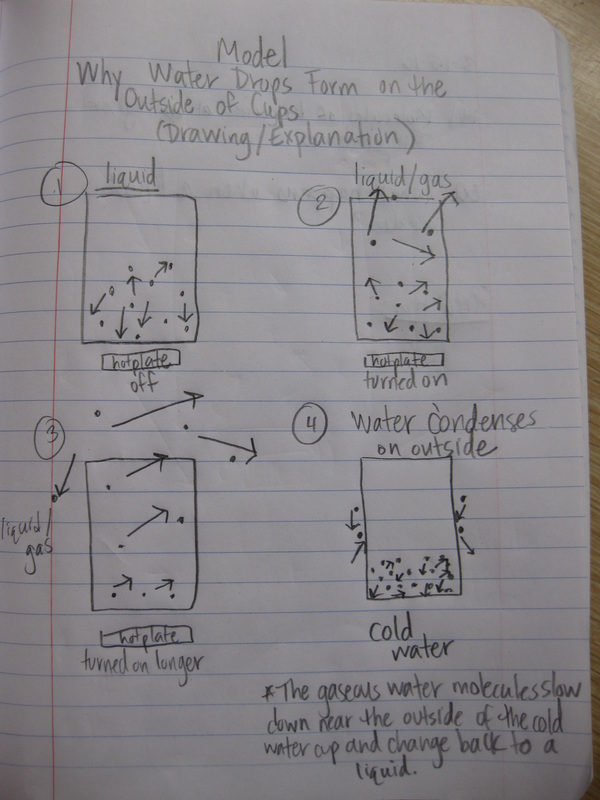
While students understand the connections between speed of molecules and temperature, how can these ideas be connected to explaining a more complex process like condensation? It was obvious for students to see that as the temperature of a hot plate increased, water began to evaporate and boil. We turned what we saw into a physical model (check out the video below). And then used this to explain how water that had evaporated into the air could condense on the outside of a cold cup above. Check out our models to explain this next week!
An important aspect of understanding what happens as gases are heated and cooled also relates to the air around them and its temperature. Watching two videos on how carbon dioxide warms up and cools down, the gas was contained in a rubber balloon. It's obvious from our previous knowledge that the balloon expanded and shrank. But the air around each of the balloons was doing something, too, which allowed it to do so.
|
Mrs. BrinzaThree Favorite Smells: Archives
April 2016
Categories |

















 RSS Feed
RSS Feed